| Osmosis & Diffusion – Lab 1 |  |
Introduction:
All molecules have kinetic energy and are constantly in motion. This motion causes the molecules to bump into each other and move in different directions. The result is diffusion. Diffusion is the random movement of molecules from an area of high concentration to an area of low concentration. This will continue until dynamic equilibrium is reached; no net movement will occur. Osmosis is a special kind of diffusion. It is the diffusion of water through a selectively permeable membrane. A selectively permeable membrane means that the membrane will only allow certain molecules through such as water, small solutes, oxygen, carbon dioxide, and glucose, because no additional ATP is required. The membrane will not let ions, nonpolar molecules, or large molecules through because extra ATP is needed for them to travel across the membrane. Active transport is how molecules (such as ions) move against the concentration gradient. Additional ATP is required to perform this process.
Water will travel from an area of high water potential to an area of low water potential. Water potential is the measure of free energy of water in a certain solution. It is measured by using the Greek letter psi (ψ). The formula for figuring water potential is:
ψ = ψp + ψs
Water Potential = Pressure Potential + Solute Potential
Water potential is affected by 2 different factors. They are the addition of a solute and the pressure potential. If a solute is added to the water, then the water potential is lowered. If more pressure is placed on the water, then the potential is raised. The addition of a solute and water potential are inversely proportional. Pressure being placed onto the water and the potential of the water are directly proportional.
Solutions can have three relationships with each other; isotonic, hypertonic, or hypotonic. When the solutions have the same concentration of solutes, they are isotonic. There is no net change in the amount of water on each side of the membrane. If the solutions differ in their solute concentrations, the solution that has the most solute is hypertonic to the other solution. The solution with the smaller amount of solute is hypotonic to the other solution. The net movement of water will be from the hypertonic solution to the hypotonic solution. Net movement will occur until dynamic equilibrium is reached, then there will be no net movement of water.
Hypothesis:
In this lab, osmosis and diffusion will occur between the solutions of different concentration until dynamic equilibrium is reached and there is no net movement of water.
Materials:
Exercise 1A:
The materials used include a 30cm piece of 2.5cm dialysis tubing, string, scissors, 15mL of 15% glucose/1% starch solution, 250mL beaker, distilled water, and 4mL of Lugol’s solution (Iodine Potassium-Iodine or IKI).
Exercise 1B:
This exercise required six 30cm strips of presoaked dialysis tuning, six 250mL cups or beakers, string, scissors, a balance, and 25mL of these solutions: distilled water, 0.2M sucrose, 0.4M sucrose, 0.6M sucrose, 0.8M sucrose, and 1.0M sucrose.
Exercise 1C:
The materials that were required include 100mL of these solutions: distilled water, 0.2M sucrose, 0.4M sucrose, 0.6M sucrose, 0.8M sucrose, and 1.0M sucrose, six 250mL beakers or cups, a potato, a cork borer, a balance, paper towel, and plastic wrap.
Exercise 1D:
The materials used include a calculator, and a pencil.
Procedure:
Exercise 1A:
Soak the dialysis tubing in water. Tie off one end of the tubing to form a bag. Open the bag and place the glucose/starch solution in it. Tie off the other end of the bag, leaving enough room for expansion of the contents in the bag. Record the color of the solution in Table 1.1. Next, test the glucose/starch solution for the presence of glucose. Record the results in Table 1.1. Fill a 250mL beaker or cup with 2/3 full with distilled water. Add 4mL of Lugol’s solution to the distilled water and record the color of the solution in Table 1.1. Test the solution for glucose and record the results in Table 1.1. Immerse the bag in the beaker of solution. Allow the beaker and bag to stand for approximately 30 minutes or until you see a distinct color change in the bag and the beaker. Record the final color of the solution in the bag, and the solution in the beaker, in Table 1.1. Test the liquid in the beaker and in the bag for the presence of glucose. Record the results in Table 1.1.
Exercise 1B:
Obtain the six strips of presoaked dialysis tubing and create a bag out of each one by tying off one end. Pour 25mL of the 6 solutions into separate bags. Tie off the other end of the 6 bags. Rinse each bag gently with distilled water and blot dry. Determine the mass of each bag and record it in Table 1.2. Immerse each bag in one beaker filled will distilled water and label the beaker to indicate the molarity of the solution in the bag. Let the setups stand for 30 minutes. Remove the bags from the water. Carefully blot them dry and determine their masses. Record them in Table 1.2. Obtain the other lab groups data to complete Table 1.3.
Exercise 1C:
Pour 100mL of the solutions into a labeled 250mL beaker. Use a cork borer to cut potato cylinders. You need 4 cylinders for each cup. Determine the mass of the 4 cylinders together and record the amount in Table 1.4. Place the cylinders into the beaker of sucrose solution. Cover the beaker with plastic wrap to prevent evaporation. Let it stand overnight. Remove the cores from the beaker and blot them gently on a paper towel and determine their total mass. Record the results in Table 1.4. Calculate the percentage change. Do this for the individual and class data. Graph the class average percentage change in mass.
Exercise 1D:
Determine the solute, pressure, and water potential of the sucrose solution. Then, graph the information that is given about the zucchini cores.
Results:
Exercise 1A:
Table 1.1
| Initial Contents | Initial Color | Final Color | Initial Presence of Glucose | Final Presence of Glucose | |
| Bag | 15% glucose & 1% starch | Cloudy White | Purple | Yes | Yes |
| Beaker | Water & IKI | Brown | Orange | No | Yes |
- Which substances are entering the bag and which are leaving the bag? What evidence supports the answer? Distilled water and IKI are leaving and entering. Glucose is able to leave the bag.
- Explain the results that were obtained. Include the concentration differences and membrane pore size in the discussion. Glucose and small molecules were able to move through the pores. Water and IKI moved from high to low concentration.
- How could this experiment be modified so that quantitative data could be collected to show that water diffused into the dialysis bag? You could mass the bag before and after it was placed into the solution.
- Based on your observations, rank the following by relative size, beginning with the smallest: glucose molecules, water molecules, IKI molecules, membrane pores, and starch molecules. Water molecules, IKI molecules, Glucose molecules, Membrane pores, and Starch molecules
- What results would you expect if the experiment started with a glucose and IKI solution inside the bag and only starch and water outside? The glucose and IKI would move out of the bag and turn the starch and water solution purple/blue. The starch couldn’t move inside the bag because its molecules are too big to pass through the membrane of the tubing.
Exercise 1B:
Table 1.2: Dialysis Bag Results: Individual Data
| Contents in dialysis bag | Initial mass (g) | Final mass (g) | Mass difference (g) | % Change in mass |
| Distilled Water | 24.7 | 23.7 | 1 | 4.1 |
| 0.2M | 26.7 | 27.4 | .7 | 2.62 |
| 0.4M | 27.4 | 29 | 1.6 | 5.84 |
| 0.6M | 25.9 | 29 | 3.1 | 12 |
| 0.8M | 29 | 32.6 | 3.6 | 12.41 |
| 1.0M | 28 | 33.7 | 5.7 | 20.4 |
Table 1.3: Dialysis Bag Results: Class Data
| Group 1 |
Group 2 |
Group 3 |
Total | Class Average | |
| Distilled Water | 4.1% | .7% | 1.6% | 6.4% | 2.13% |
| 0.2M | 2.62% | 6.4% | 4.1% | 13.12% | 4.37% |
| 0.4M | 5.84% | 9.9% | 9.5% | 25.24% | 8.41% |
| 0.6M | 12% | 13.4% | 9.3% | 34.37% | 11.57% |
| 0.8M | 12.41% | 14.6% | 15.2% | 42.21% | 14.07% |
| 1.0M | 20.4% | 19.7% | 15.9% | 56% | 18.67% |
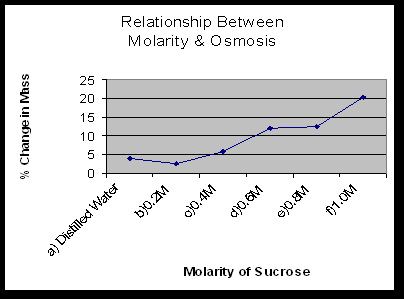
- Explain the relationship between the change in mass and the molarity of sucrose within the dialysis bags. The solute is hypertonic and water will move into the bag. As the molarity increases the water moves into the bag.
- Predict what would happen to the mass of each bag in this experiment if all the bags were placed in a 0.4M sucrose solution instead of distilled water. Explain. With the 0.2M bag, the water would move out. With the 0.4M bag, there will be no net movement of water because the solutions reach dynamic equilibrium. With the 0.6M-1M bags, the water would move into the bag.
- Why did you calculate the percent change in mass rather than simply using the change in mass? This was calculated because each group began with different initial masses and we would have different data. All the groups needed consistent data.
- A dialysis bag is filled with distilled water and then places in a sucrose solution. The bag’s initial mass is 20g and its final mass is 18g. Calculate the percent change of mass, showing your calculations. ((18-20)/20) x 100 = 10%
- The sucrose solution in the beaker would have been hypotonic to the distilled water in the bag.
Exercise 1C
Table 1.4: Potato Core: Individual Data
| Contents of Beaker | Initial Mass (g) | Final Mass (g) | Difference in Mass | % Change in Mass |
| Distilled Water | 2.8 | 3.7 | .9 | 32.14 |
| 0.2M | 2.9 | 3.1 | .2 | 7 |
| 0.4M | 2.5 | 2.2 | .3 | 12 |
| 0.6M | 2.3 | 1.9 | .4 | 17.39 |
| 0.8M | 2.5 | 1.9 | .6 | 24 |
| 1.0M | 2.3 | 1.8 | .5 | 21.74 |
Table 1.5: Potato Core: Class Data
| Group 1 | Group 2 | Total | Class Average | |
| Distilled Water | 32.14% | 21.1% | 53.24% | 26.62% |
| 0.2M | 7% | 6.7% | 13.7% | 6.85% |
| 0.4M | -12% | -6.5% | -18.5% | -9.25% |
| 0.6M | -17.39% | -15.2% | -32.59% | -16.30% |
| 0.8M | -24% | -20% | -44% | -22% |
| 1.0M | -21.74% | -19% | -40.74% | -20.37% |
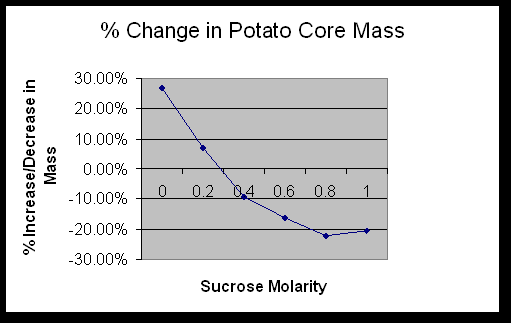
Determine the molar concentration of the potato core. 0.3M
Exercise 1D
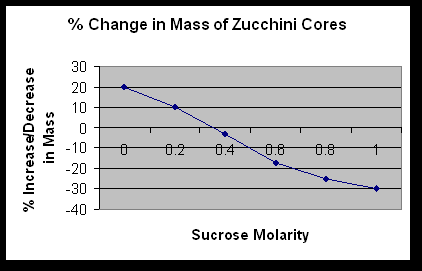
What is the molar concentration of the zucchini cores? .35M
- If a potato core is allowed to dehydrate by sitting in the open air, would the water potential of the potato cells decrease or increase? Why? It would decrease because the water would leave the cells and cause the water potential to go down.
- If a plant cell has a lower water potential than its surrounding environment and if pressure is equal to zero, is the cell hypertonic or hypotonic to its environment? Will the cell gain water or lose water? It is hypotonic and it will gain water.
- The beaker is open to the atmosphere. What is the pressure potential of the system? The pressure potential is zero.
- Where is the greatest water potential? In the dialysis bag.
- Water will diffuse out of the bag. Why? It is because the water moves from and area of high water potential to an area of lower water potential.
- What effect does adding solute have on the solute potential component of that solution? Why? It makes is more negative.
- Consider what would happen to a red blood cell placed in distilled water: a) Which would have the higher concentration of water molecules? Distilled Water b) Which would have the higher water potential? Distilled Water c) What would happen to the red blood cell? Why? It would lyce, because it would take on too much water.
Error Analysis:
Possible errors that could have affected the results of the lab include incorrectly mixing the solutions, ineffectively tying the dialysis tubing, inaccurately measuring , and inaccurately calculating.
Conclusion:
During Exercise 1A the data that was collected help determine which molecules can and can not move across a cell membrane. Obviously, because of the color change in the bag, the IKI was able to move across the membrane. It is small enough to fit through the pores in the selectively permeable membrane, along with water. Starch was too large to move across the membrane. Glucose, as the Benedict’s test proves, was able to move freely along with the water and IKI solution.
In Exercise 1B, it was proven that water moves faster across the cell membrane than sucrose. The water moved to help reach dynamic equilibrium between the 2 solutions. The sucrose molecules are too big to move across the membrane as fast as water can.
The data in Exercise 1C showed that the potatoes contained sucrose. The sucrose in the potato raised the solute potential, which lowered the water potential. The beaker of distilled water had a high water potential. Water moves down the concentration gradient, causing the potato cores to take on water.
Exercise 1D helped better understand the lab with simple algebra equations. It proved that the data that was collected was correct through mathematics.
