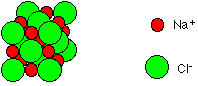| Biochemistry | All Materials © Cmassengale |
I. Cells Contain Organic Molecules
A. Most Common Elements
1. Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen.
2. These four elements constitute about 95% of your body weight.
3. Chemistry of carbon allows the formation of an enormous variety of organic molecules.
4. Organic molecules have carbon and hydrogen; determine structure and function of living things.
5. Inorganic molecules do not contain carbon and hydrogen together; inorganic molecules (e.g., NaCl) can play important roles in living things.
B. Small Molecules Have Functional Groups
1. Carbon has four electrons in outer shell; bonds with up to four other atoms (usually H, O, N, or another C).

2. Ability of carbon to bond to itself makes possible carbon chains and rings; these structures serve as the backbones of organic molecules.
3. Functional groups are clusters of atoms with characteristic structure and functions.
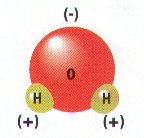
a. Polar molecules (with +/- charges) are attracted to water molecules and are hydrophilic.
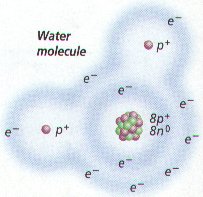
b. Nonpolar molecules are repelled by water and do not dissolve in water; are hydrophobic.
c. Hydrocarbon is hydrophobic except when it has an attached ionized functional group such as carboxyl (acid) ( COOH); then molecule is hydrophilic.
d. Cells are 70-90% water; degree organic molecules interact with water affects their function.
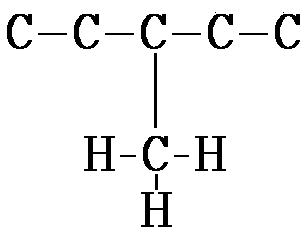
4. Isomers are molecules with identical molecular formulas but differ in arrangement of their atoms
|  |
C. Large Organic Molecules Have Monomers
1. Each small organic molecule can be a unit of a large organic molecule called a macromolecule.
2. Small organic molecules (e.g., monosaccharides, glycerol and fatty acid, amino acids, and nucleotides) that can serve as monomers, the subunits of polymers.
3. Polymers are the large macromolecules composed of three to millions of monomer subunits.
4. Four classes of macromolecules (polysaccharides or carbohydrates, triglycerides or lipids, polypeptides or proteins, & nucleic acids such as DNA & RNA) provide great diversity.
D. Condensation Is the Reverse of Hydration
1. Macromolecules build by different bonding of different monomers; mechanism of joining and breaking these bonds is condensation and hydrolysis.
2. Cellular enzymes carry out condensation and hydrolysis of polymers.
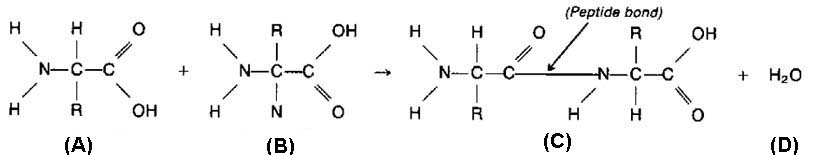
3. Condensation involves a dehydration synthesis because a water is removed (dehydration) and a bond is made (synthesis).
a. When two monomers join, a hydroxyl ( OH) group is removed from one monomer and a hydrogen is removed from the other.
b. This produces the water given off during a condensation reaction.
4. Hydrolysis (hydration) reactions break down polymers in reverse of condensation; a hydroxyl
( OH) group from water attaches to one monomer and hydrogen ( H) attaches to the other.
II. Carbohydrates
A. Monosaccharides, Disaccharides, and Polysaccharides
1. Monosaccharides are simple sugars with a carbon backbone of three to seven carbon atoms.
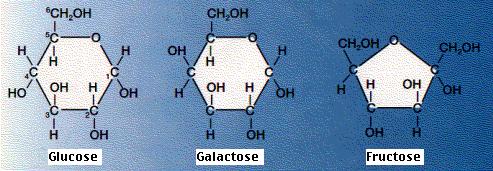
a. Best known sugars have six carbons (hexoses).
![[Glucose Straight Structure]](https://biologyjunction.com/images/glucose1.jpg)
1) Glucose and fructose isomers have same formula (C6H12O6) but differ in structure.
2) Glucose is commonly found in blood of animals; is immediate energy source to cells.
3) Fructose is commonly found in fruit.
4) Shape of molecules is very important in determining how they interact with one another.
2. Ribose and deoxyribose are five-carbon sugars (pentoses); contribute to the backbones of RNA and DNA, respectively.
3. Disaccharides contain two monosaccharides joined by condensation.
a. Sucrose is composed of glucose and fructose and is transported within plants.
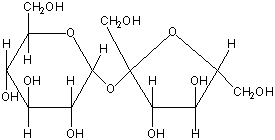
b. Lactose is composed of galactose and glucose and is found in milk.
c. Maltose is two glucose molecules; forms in digestive tract of humans during starch digestion.

| Sugar | Sweetness |
|---|---|
| fructose | 173% |
| sucrose | 100% |
| glucose | 74% |
| maltose | 33% |
| galactose | 33% |
| lactose | 16% |
B. Polysaccharides Are Varied in Structure and Function
1. Polysaccharides are chains of glucose molecules or modified glucose molecules
a. Starch is straight chain of glucose molecules with few side branches.

b. Glycogen is highly branched polymer of glucose with many side branches; called “animal starch,” it is storage carbohydrate in the liver of animals.

c. Cellulose is glucose bonded to form microfibrils; primary constituent of plant cell walls.
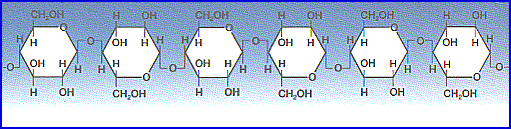
d. Chitin is polymer of glucose with amino acid attached to each; it is primary constituent of crabs and related animals like lobsters and insects.
III. Lipids
A. Lipids
1. Lipids are varied in structure.
2. Many are insoluble in water because they lack polar groups.
B. Fats and Oils Are Similar
1. Each fatty acid is a long hydrocarbon chain with a carboxyl (acid) group at one end.
a. Because the carboxyl group is a polar group, fatty acids are soluble in water.
b. Most fatty acids in cells contain 16 to 18 carbon atoms per molecule.
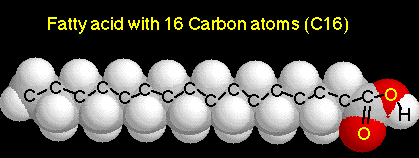
c. Saturated fatty acids have no double bonds between their carbon atoms. (C-C-C-)
d. Unsaturated fatty acids have double bonds in the carbon chain.(C-C-C-C=C-C-)

e. Saturated animal fats are associated with circulatory disorders; plant oils can be substituted for animal fats in the diet.
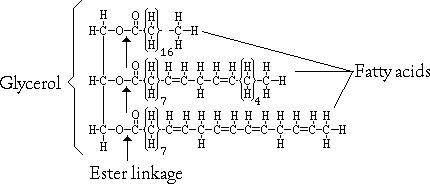
2. Glycerol is a water-soluble compound with three hydroxyl groups.
3. Triglycerides are glycerol joined to three fatty acids by condensation
4. Fats are triglycerides containing saturated fatty acids (e.g., butter is solid at room temperature).
5. Oils are triglycerides with unsaturated fatty acids (e.g., corn oil is liquid at room temperature).
6. Fats function in long-term energy storage in organisms; store six times the energy as glycogen.
C. Waxes Are Nonpolar Also
1. Waxes are a long-chain fatty acid bonded to a long-chain alcohol.
a. Solid at room temperature; have a high melting point; are waterproof and resist degradation.
b. Form protective covering that retards water loss in plants; maintain animal skin and fur.
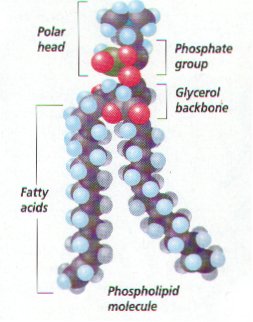
D. Phospholipids Have a Polar Group
1. Phospholipids are like neutral fats except one fatty acid is replaced by phosphate group or a group with both phosphate and nitrogen
![[Lecithin]](https://biologyjunction.com/images/lecithin.jpg)
2.Phosphate group is the polar head: hydrocarbon chain becomes nonpolar tails
3. Phospholipids arrange themselves in a double layer in water, so the polar heads face outward toward water molecules and nonpolar tails face toward each other away from water molecules.
![[Phospholipid Bilayer]](https://biologyjunction.com/images/membrane.jpg)
4. This property enables them to form an interface or separation between two solutions (e.g., the interior and exterior of a cell); the plasma membrane is a phospholipid bilayer.
E. Steroids Have Carbon Rings
1. Steroids differ from neutral fats; steroids have a backbone of four fused carbon rings; vary according to attached functional groups.
2. Cholesterol is a precursor of other steroids, including aldosterone and sex hormones.
3. Testosterone is the male sex hormone.
4. Functions vary due primarily to different attached functional groups.
IV. Proteins
A. Amino Acids

1. Amino acids are the monomers that condense to form proteins, which are very large molecules with structural and metabolic functions.
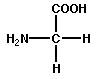
2. Structural proteins include keratin, which makes up hair and nails, and collagen fibers, which support many organs.
3. Myosin and actin proteins make up the bulk of muscle.
4. Enzymes are proteins that act as organic catalysts to speed chemical reactions within cells.
5. Insulin protein is a hormone that regulates glucose content of blood.
6. Hemoglobin transports oxygen in blood.
7. Proteins embedded in the plasma membrane have varied enzymatic and transport functions.
B. Peptide Bonds Join Amino Acids
1. All amino acids contain a carboxyl (acid) group ( COOH) and an amino group ( NH2).
2. Both ionize at normal body pH to produce COO- and NH+; thus, amino acids are hydrophilic.
3. Peptide bond is a covalent bond between amino acids in a peptide; results from condensation reaction.
a. Atoms of a peptide bond share electrons unevenly (oxygen is more electronegative than nitrogen).
b. Polarity of the peptide bond permits hydrogen bonding between parts of a polypeptide.
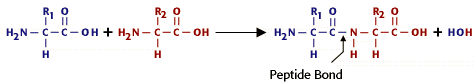
4. Amino acids differ in nature of R group, ranging from single hydrogen to complicated ring compounds.
a. R group of amino acid cysteine ends with a sulfhydryl ( SH) that serves to connect one chain of amino acids to another by a disulfide bond ( S S).
b. There are 20 different amino acids commonly found in cells.
5. A peptide is two or more amino acids joined together.
a. Polypeptides are chains of many amino acids joined by peptide bonds.
b. Protein may contain more than one polypeptide chain; it can have large numbers of amino acids.
C. Proteins Can Be Denatured
1. Both temperature and pH can change polypeptide shape.
a. Examples: heating egg white causes albumin to congeal; adding acid to milk causes curdling. When such proteins lose their normal configuration, the protein is denatured.
b. Once a protein loses its normal shape, it cannot perform its usual function.
2. The sequence of amino acids, therefore, forecasts the protein’s final shape.
D. Proteins Have Levels of Structure
1. Final 3-D shape of a protein determines function of the protein in the organism.
a. Primary structure is sequence of amino acids joined by peptide bonds.
1) Frederick Sanger determined first protein sequence, with hormone insulin, in 1953.
a) First broke insulin into fragments and determined amino acid sequence of fragments.
b) Then determined sequence of the fragments themselves.
c) Required ten years research; modern automated sequencers analyze sequences in hours.
2) Since amino acids differ by R group, proteins differ by a particular sequence of the R groups.
b. Secondary structure results when a polypeptide takes a particular shape.
1) The (alpha) helix was the first pattern discovered by Linus Pauling and Robert Corey.
a) In peptide bonds, oxygen is partially negative, hydrogen is partially positive.
b) Allows hydrogen bonding between the C O of one amino acid and the N H of another.
c) Hydrogen bonding between every fourth amino acid holds spiral shape of a helix.
d) helices covalently bonded by disulfide (S S) linkages between two cysteine amino acids.
2) The sheet was the second pattern discovered.
a) Pleated sheet polypeptides turn back upon themselves; hydrogen bonding occurs between extended lengths.
b) keratin includes keratin of feathers, hooves, claws, beaks, scales, and horns; silk also is protein with sheet secondary structure.
3. Tertiary structure results when proteins of secondary structure are folded, due to various interactions between the R groups of their constituent amino acids
4. Quaternary structure results when two or more polypeptides combine.
1) Hemoglobin is globular protein with a quaternary structure of four polypeptides.
2) Most enzymes have a quaternary structure.
V. Nucleic Acids
A. Nucleotides
1. Nucleotides are a molecular complex of three types of molecules: a phosphate (phosphoric acid), a pentose sugar, and a nitrogen-containing base.
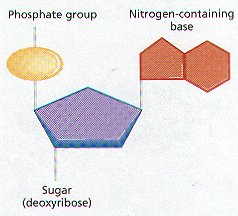
2. Nucleotides have metabolic functions in cells.
a. Coenzymes are molecules, which facilitate enzymatic reactions.
b. ATP (adenosine triphosphate) is a nucleotide used to supply energy.
c. Nucleotides also serve as nucleic acid monomers.
B. Nucleic Acids
1. Nucleic acids are huge polymers of nucleotides with very specific functions in cells.
2. DNA (deoxyribonucleic acid) is the nucleic acid whose nucleotide sequence stores the genetic code for its own replication and for the sequence of amino acids in proteins.
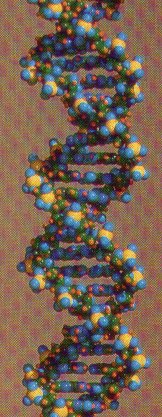
3. RNA (ribonucleic acid) is a single-stranded nucleic acid that translates the genetic code of DNA into the amino acid sequence of proteins.
4. DNA and RNA differ in the following ways:
a. Nucleotides of DNA contain deoxyribose sugar; nucleotides of RNA contain ribose.
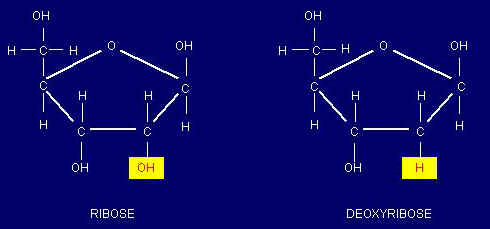
b. In RNA, the base uracil occurs instead of the base thymine, as in DNA.
c. DNA is double-stranded with complementary base pairing; RNA is single-stranded.
1) Complementary base pairing occurs where two strands of DNA are held together by hydrogen bonds between purine and pyrimidine bases
2) The number of purine bases always equals the number of pyrimidine bases; called Chargaff’s rule
3) Adenine pairs with Thymine & guanine pairs with cytoseine on DNA
4) Guanine & adenine are purines; Cytosine & thymine are pyrimidines
d. Two strands of DNA twist to form a double; RNA generally does not form helices.
C. ATP (Adenosine Triphosphate)
1. ATP (adenosine triphosphate) is a nucleotide of adenosine composed of ribose and adenine.
2. Derives its name from three phosphates attached to the five-carbon portion of the molecule.
3. ATP is a high-energy molecule because the last two unstable phosphate bonds are easily broken.
4. Usually in cells, a terminal phosphate bond is hydrolyzed, leaving ADP (adenosine diphosphate).
5. ATP is used in cells to supply energy for energy-requiring processes (e.g., synthetic reactions); whenever a cell carries out an activity or builds molecules, it “spends” ATP.

Summary of Biological Macromolecules:
| Macromolecule | Building Blocks | Functions |
| Polysaccharides | Sugars (monosaccharides) |
|
| Lipids (Triglycerides) | Fatty acids, glycerol |
|
| Lipids (Phospholipids) | Fatty acids, glycerol, phosphate group |
|
| Proteins | Amino acids (20 types) |
|
| Nucleic Acids: DNA (forms a double helix) |
|
|
| Nucleic Acids (RNA)
3 types:
(usually a single strand) |
| Protein synthesis:
|
| BACK |