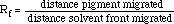Chromatography Lab
Problem: How do you separate the different pigments in a plant?
Materials:
Cone-type (size 4) coffee filter paper (or Whatman #1 chromatography paper)
large glass jars
acetone
distilled water
capillary tubes
fresh spinach
mortar and pestle
clean sand
Introduction:
In this activity you will be experimenting with a technique called chromatography which will allow you to visually demonstrate that the pigment in leaves is a combination of several different colored pigments.
This technique is useful in that it can separate and identify the various components of mixtures, such as those contained in plant pigments. A pigment is a substance that absorbs light at specific wavelengths, chlorophyll is one of these pigments. Its green-yellow in color is due to the absorption of red, orange, blue, and violet wavelengths and the reflection of the green and yellow wavelengths. This occurs when white light (containing all of the light wavelengths, or the entire spectrum of colors) shines on the leaf surface, all of the wavelengths are absorbed except for the ones you see, which are green-yellow, those are the portions of the spectrum being reflected.
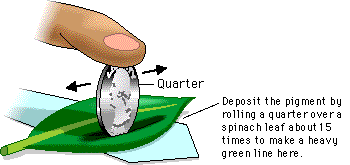
If the conditions are identical, the relative distance moved by a particular compound is the same from one mixture to another. This is why chromatography can be used to identify a compound. The actual identification requires a simple calculation as shown below:
Rf = distance moved by compound from original spot divided by the distance moved by solvent from original spot
It is important to remember that several factors can influence the reliability of the Rf value, these include humidity, temperature, solvent, pigment extract preparation, and the amounts of the material present. Values are comparable only when the extracts are prepared in the same way and the chromatograms are prepared identically and developed together in the same container.
Acetone is flammable (even the amount found in nail polish remover), keep it away from sparks or open flames. Wear eye protection, especially if using pure acetone.
Procedure
1. Each lab group (or individual if not working in groups) will need 4 strips of filter paper, approximately 6 inches long and 1 inch wide, 2 chromatography development containers (500 ml beakers or large fruit jars work well), 2 large rubber bands (able to stretch around the vessels from the mouth to the bottom of the vessel), 2 solvents, water and either pure acetone, or nail polish remover.
2. Do the following with both fresh spinach leaves; tear leaf material and place in a glass container, cover with acetone (this should be done the day before the actual lab activity). An alternative pigment extraction technique is to use a
mortar and pestle. Place plant material the vessel, add a little clean sand, some acetone and then grind until a dark green liquid appears. Both techniques yield very dark pigments with which to work. Be certain to keep the pigments apart throughout the entire activity.
3. Place one of each solvents (water and acetone, or nail polish remover) in the chromatography vessels and stretch a rubber band length-wise around each vessel. The rubber band will be the mechanism for hanging the chromatography strips.
4. Make a pencil mark on each of the 2 chromatography strips, in the center, directly above the point of the strip, about 1 inch from the tip of the paper. Using a capillary tube, or tooth pick, apply the plant pigment to each filter paper strip. This is done by touching the tooth pick or capillary tube which has been dipped in the pigment, to the pencil mark. Make an application, then wave the paper gently to dry it a little before the next application. Be patient, you will need 12 to 15 applications.
5. By now you should have 2 strips with spinach pigment. Suspend one of each in each of the chromatography development vessels. You can attach them with paper clips, or simply fold over a portion of the end and it should hang in place. The tip of each strip should just touch the solvent.
6. Wait 20 to 30 minutes for the chromatograms to develop. Remove the chromatograms. Mark with a pencil (NOT a pen) where the solvent stopped as it moved up the chromatogram. This is called the solvent front. Mark also where each pigment stopped moving up the chromatogram. Using the equation below, determine a reference number for each pigment on the chromatograms. Depending on which chromatogram you are viewing, you should see greens, yellow/yellow orange, and red. All measurements should be in mm. (Any material which did not move from the
pencil dot is insoluble).
Rf = distance moved by compound from original spot divided by the
distance moved by solvent from original spot
Note: each pigment has a special name,
green = chlorophyll a or b
yellow/yellow orange = carotene
red = anthocyanin
brown = xanthophyll
The reference numbers for the chlorophylls in this activity are:
0.28 = chlorophyll a, 0.18 = chlorophyll b (spinach). You need these
numbers so that you can determine one chlorophyll from the other.
Calculate reference fronts for all of your pigments.
See if your calculations come close to those above for chlorophyll a and b.
Note: You can use different solvents such as mixtures involving petroleum ether
to do this sort of paper chromatography.
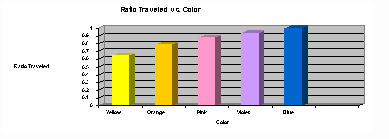
To view notes and a graphic showing a separation of plant pigments involving
paper chromatography, click here. Can you calculate the Rf values for the
pigments separated in this graphic?
Conclusion Questions
1. What reference numbers (Rf) did you calculate for chlorophyll a and chlorophyll b?
2. With what you have discovered about pigments, what conclusions can you
make regarding the changing color of leaves in autumn?
3. What adaptive purpose do different colored pigments serve for a plant?
4. Why do some pigments move farther up the chromatogram than others?
5. What are some possible sources of error in this lab?
Paper chromatography is a technique used to separate a mixture into its component molecules. The molecules migrate, or move up the paper, at different rates because of differences in solubility, molecular mass, and hydrogen bonding with the paper.
For a simple, beautiful example of this technique, draw a large circle in the center of a piece of filter paper with a black water-soluble, felt-tip pen. Fold the paper into a cone and place the tip in a container of water. In just a few minutes you will have tie-dyed filter paper!
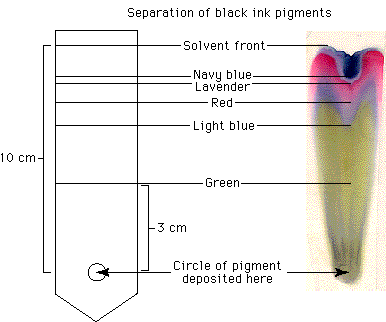
The green, blue, red, and lavender colors that came from the black ink should help you to understand that what appears to be a single color may in fact be a material composed of many different pigments —and such is the case with chloroplasts.

In paper chromatography the pigments are dissolved in a solvent that carries them up the paper. In the ink example, the solvent is water. To separate the pigments of the chloroplasts, you must use an organic solvent
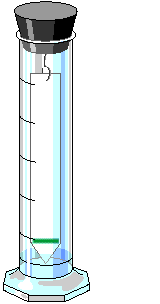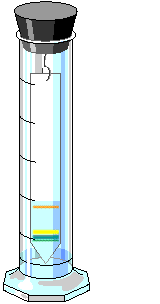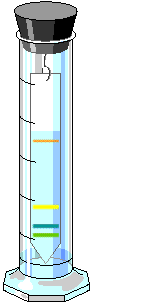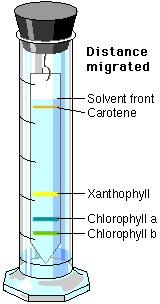

If you did a number of chromatographic separations, each for a different length of time, the pigments would migrate a different distance on each run. However, the migration of each pigment relative to the migration of the solvent would not change. This migration of pigment relative to migration of solvent is expressed as a constant, Rf (Reference front). It can be calculated by using the formula: