Enzyme Catalysis
Introduction
Enzymes are proteins produced by living cells that act as catalysts, which affect the rate of a biochemical reaction. They allow these complex biochemical reactions to occur at a relatively low temperature and with less energy usage.
In enzyme-catalyzed reactions, a substrate, the substance to be acted upon, binds to the active site on an enzyme to form the desired product. Each active site on the enzyme is unique to the substrate it will bind with causing each to have an individual three-dimensional structure. This reaction is reversible and is shown as following:
E + S—-ES—- E + P
Enzymes are recyclable and unchanged during the reaction. The active site is the only part of the enzyme that reacts with the substrate. However, its unique protein structure under certain circumstances can easily be denatured. Some of the factors that affect enzyme reactions are salt concentration, pH, temperature, substrate and product concentration, and activators and inhibitors.
Enzymes require a very specific environment to be affective. Salt concentration must be in an intermediate concentration. If the salt concentration is too low, the enzyme side chains will attract each other and form an inactive precipitate. Likewise, if the salt concentration is too high, the enzyme reaction is blocked by the salt ions. The optimum pH for an enzyme-catalyzed reaction is neutral (7 on the pH scale). If the pH rises and becomes basic, the enzyme begins losing its H+ ions, and if it becomes too acidic, the enzyme gains H+ ions. Both of these conditions denature the enzyme and cause its active site to change shape.
Enzymes also have a temperature optimum, which is obtained when the enzyme is working at its fastest, and if raised any further, the enzyme would denature. For substrate and product concentrations, enzymes follow the law of mass action, which says that the direction of a reaction is directly dependent on the concentration. Activators make active sites better fit a substrate causing the reaction rate to increase. Inhibitors bind with the enzymes’ active site and block the substrate from bonding causing the reaction to subside.
The enzyme in this lab is catalase, which produced by living organisms to prevent the accumulation of toxic hydrogen peroxide. Hydrogen peroxide decomposes to form water and oxygen as in the following equation:
2H2O2 ® 2H2O + O2
This reaction occurs spontaneously without catalase, but the enzyme speeds the reaction considerably. This lab’s purpose is to prove that catalase does speed the decomposition of hydrogen peroxide and to determine the rate of this reaction.
Hypothesis
The enzyme catalase, under optimum conditions, effectively speeds the decomposition of hydrogen peroxide.
Materials
Exercise 2A: Test of Catalase Activity
In Part 1, the materials used were 10mL of 1.5% H2O2, 50-mL glass beaker, 1 mL catalase, and 2 10-mL pipettes and pipette pumps. In Part 2, the materials used were 5 mL of catalase, a boiling water bath, 1 test tube, a test tube rack, 10 mL of 1.5% H2O2, 50-mL beaker, and 2 10-mL pipettes and pipette pumps. In Part 3, the materials used were 10 mL of 1.5% H2O2, 50-mL beaker, liver, and a syringe.
Exercise 2B: The Baseline Assay
This part of the lab required 10 mL of 1.5% H2O2, 1 mL distilled H2O, 10 mL of H2SO4, 2 50-mL beakers, a sheet of white paper, 5 mL KMnO4, 2 5-mL syringes, and 2 10-mL pipettes and pumps.
Exercise 2C: The Uncatalyzed Rate of H2O2 Decomposition
The materials used for this section were 15 mL of 1.5% H2O2, 1 mL distilled H2O, 10 mL H2SO4, 2 50-mL beakers, a sheet of white paper, 5 mL KMnO4, 2 5-mL syringes, and 2 10-mL pipettes and pumps.
Exercise 2D: An Enzyme-Catalyzed Rate of H2O2 Decomposition
The materials required for Exercise 2D were 70 mL of 1.5% H2O2, 70 mL of H2SO4, 6 mL of catalase solution, 13 plastic, labeled cups, 3 100-mL beakers, 1 50-mL beaker, 1 10-mL syringe, 1 5-mL syringe, 1 60-mL syringe, a sheet of white paper, a timer, and 30 mL of KMnO4.
Method
Exercise 2A: Test of Catalase Activity
In Part 1, 10 mL of 1.5% H2O2 were transferred into a 50-mL beaker. Then, 1 mL of fresh catalase solution was added and the reaction was observed and recorded. In Part 2, 5 mL of catalase was placed in a test tube and put in a boiling water bath for five minutes. 10 mL of 1.5% H2O2 were transferred to a 50-mL beaker and 1 mL of the boiled catalase was added. The reaction was observed and recorded. In Part 3, 10mL of 1.5% H2O2 were transferred to a 50 mL beaker. 1 cm3 of liver was added to the beaker and the reaction was observed and recorded.
Exercise 2B: The Baseline Assay
10 mL of 1.5% H2O2 were transferred to a 50-mL beaker. 1 mL of H2O was added instead of catalase, and then, 10 mL of H2SO4 were added. After mixing well, a 5 mL sample was removed and placed over a white sheet of paper. A 5-mL syringe was used to add KMnO4, 1 drop at a time until a persistent brown or pink color was obtained. The solution was swirled after every drop, and the results were observed and recorded. The baseline assay was calculated.
Exercise 2C: The Uncatalyzed Rate of H2O2 Decomposition
A small quantity of H2O2 was placed in a beaker and stored uncovered for approximately 24 hours. To determine the amount of H2O2 remaining, 10 mL of 1.5% H2O2 were transferred to a 50-mL beaker. 1 mL of H2O was added instead of catalase, and then, 10 mL of H2SO4 were added. After mixing well, a 5 mL sample was removed and placed over a white sheet of paper. A 5-mL syringe was used to add KMnO4, 1 drop at a time until a persistent brown or pink color was obtained. The solution was swirled after every drop, and the results were observed and recorded. The percent of the spontaneously decomposed H2O2 was calculated.
Exercise 2D: An Enzyme-Catalyzed Rate of H2O2 Decomposition
The baseline assay was reestablished following the directions of Exercise 2B. Before starting the actual experiment a lot of preparation was required. Six labeled cups were set out according to their times and 10 mL of H2O2 were added to each cup. 6 mL of catalase were placed in a 10-mL syringe, and 60 mL of H2SO4 were placed in a 60-mL syringe. To start the actual lab, 1 mL of catalase was added to each of the cups, while simultaneously, the timer was started. Each of the cups were swirled. At 10 seconds, 10 mL of H2SO4 were added to stop the reaction. The same steps were repeated for the 30, 60, 120, 180, and 360 second cups, respectively.
Afterwards, a five 5 mL sample of each of the larger cups were moved to the corresponding labeled smaller cups. Each sample was assayed separately by placing each over a white sheet of paper. A 5-mL syringe was used to add KMnO4, 1 drop at a time until a persistent brown or pink color was obtained. The solution was swirled after every drop, and the results were observed and recorded.
Results
Table 1
Enzyme Activity
| Activity | Observations |
| Enzyme activity | The solution only bubbled slightly and slowly. |
| Effect of Extreme temperature | The catalase had no reaction with the H2O2; there were no bubbles |
| Presence of catalase | The solution foamed up immediately |
Table 2
Establishing a Baseline
| Volume |
| Initial reading | 5.0 mL |
| Final reading | 0.8 mL |
| Baseline ( final volume – initial volume) | 4.2 mL |
Table 3
Rate of Hydrogen Peroxide Spontaneous Decomposition
| Volume |
| Initial KMnO4 | 5.0 mL |
| Final KMnO4 | 1.2 mL |
| Amount of KMnO4 used after 24 hours | 3.8 mL |
| Amount of H2O2 spontaneously decomposed
( ml baseline – ml after 24 hours) | 0.4 mL |
| Percent of H2O2 spontaneously decomposed
( ml baseline – ml after 24 hours/ baseline) | 9.52% |
Table 4
Rate of Hydrogen Peroxide Decomposition by Catalase
| Time ( Seconds) |
| 10 | 30 | 60 | 120 | 180 | 360 |
| Baseline KMnO4 | 4.0 mL | 4.0 mL | 4.0 mL | 4.0 mL | 4.0 mL | 4.0 mL |
| Initial volume KMnO4 | 5.0 mL | 5.0 mL | 5.0 mL | 5.0 mL | 5.0 mL | 5.0 mL |
| Final volume KMnO4 | 2.2 mL | 1.4 mL | 2.0 mL | 1.7 mL | 2.4 mL | 2.3 mL |
| Amount KMnO4 used
(baseline – final) | 2.8 mL | 3.6 mL | 3.0 mL | 3.3 mL | 2.6 mL | 2.7 mL |
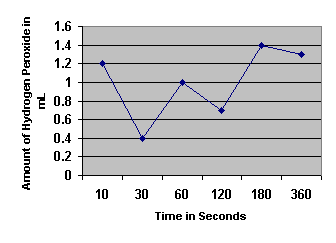
| Amount H2O2 used
(KMnO4 – initial) | 1.2 mL | 0.4 mL | 1.0 mL | 0.7 mL | 1.4 mL | 1.3 mL |
Amount of Hydrogen Peroxide Decomposed by Catalase
Exercise 2A: Test of Catalase Activity
1. Observing the reaction of catalase on hydrogen peroxide:
a. What is the enzyme in this reaction? catalase
b. What is the substrate in this reaction? Hydrogen peroxide
c. What is the product in this reaction? Oxygen & water
d. How could you show that the gas evolved is O2? The gas could be shown to be O2 if the gas were collected in a tube, and a glowing splint was held in the tube. If the splint glowed, it would prove the gas was oxygen.
2. Demonstrating the effect of boiling on enzyme action:
a. How does the reaction compare to the one using the unboiled catalase? Explain the reason for this difference. While the unboiled catalase caused bubbles to form in the solution, the boiled catalase did not react at all because boiling an enzyme causes the protein to unfold and therefore denatures it.
3. Demonstrating the presence of catalase in living tissue:
a. What do you think would happen if the potato or liver was boiled before being added to the H2O2? The catalase in the liver would have been denatured by the boiling and would not have reacted with the H2O2.
Analysis of Results
1. Determine the initial rate of the reaction and the rates between each of the time points.
| Time Intervals (Seconds) |
| Initial 0 to 10 | 10 to 30 | 30 to 60 | 60 to 120 | 120 to 180 | 180 to 360 |
| Rates | 0.12 mL/sec | -0.04 mL/sec | 0.02 mL/sec | -0.005 mL/sec | 0.01167 mL/sec | -0.00083 mL/sec |
2. When is the rate the highest? Explain why.
The rate is the highest in the initial ten seconds because the concentration of catalase is at its highest. As more of the product is formed, it blocks the reaction between the catalase and the hydrogen peroxide.
3. When is the rate the lowest? For what reasons is the rate low?
The rate is lowest during the 180-360 seconds time period because of the law of mass action. This law says that when there is a high concentration of product as in this period, the enzymes will be blocked by the product (water) from reaching and reacting with the substrate (H2O2).
4. Explain the inhibiting effect of sulfuric acid on the function of catalase. Relate this to enzyme structure and chemistry
Sulfuric acid has an inhibiting effect on catalase function because it causes the pH level in the solution to lower considerably. Acidic solutions cause the protein structure of the enzyme to gain H+ ions causing it to denature.
5. Predict the effect lowering the temperature would have on the rate of enzyme activity. Explain your prediction.
Lowering the temperature of the catalase would slow the rate of reaction until it finally caused the enzyme to denature, and it would no longer react with the substrate. Most enzymes are only affective in a temperature range between 40° – 50° C.
6. Design a controlled experiment to test the effect of varying pH, temperature, or enzyme concentration.
Part 1: Enzyme Activity at Room Temperature
Add 10 mL of 1.5% H2O2 to a 50-mL beaker, and add 1 mL of room temperature catalase. Mix well and add 10 mL of H2SO4. Watch the reaction and record the results.
Part 2: The Effect of Excessive Heat on Enzyme Activity
Put 5 mL of catalase into a test tube and heat thoroughly over a Bunsen burner. Add 1 mL of the heated catalase to 10 mL of 1.5% H2O2 in a 50-mL beaker. Add 10 mL of H2SO4. Watch the reaction and record the results.
Part 3: The Effect of Excessive Cooling on Enzyme Activity
Put 5 mL of catalase in a freezer until completely frozen. Add 1 mL of the frozen catalase to 10 mL of 1.5% H2O2 in a 50-mL beaker. Add 10 mL of H2SO4. Watch the reaction and record the results.
Error Analysis
Any number of factors in this lab could have affected the results of this experiment. To get the desired results all of the measurements had to be precisely accurate and fully planned before hand. In Exercise D especially, the factor of planning became increasingly essential. The first attempt at 2D was unsuccessful due to several reasons. First of all, the measurements, which were taken, could have possibly been inaccurate and the 60-mL syringe containing H2SO4 also dripped into one of the cups early which did not allow the reaction to fully take place. There was also some confusion on the operation of the timer and precise planning in its use. The second attempt at 2D contained errors as well. The measurements were still not as accurate as they should have been, and the solution did not appear entirely uniform. In one cup, for example, the first drop of KMnO4 left a persistent pink color, and then after over a minute, it returned back to being clear. It then took several milliliters more to get it back to a pink color.
Discussion and Conclusion
This lab showed how catalase increased the rate of decomposition of hydrogen peroxide. In 2A, it was shown that catalase causes a visual reaction with H2O2, that when boiled catalase is no longer reactive, and that catalase is present in living tissue. Lab 2C shows that the natural decomposition of H2O2 is much slower than the enzymatic reaction. Lab 2D showed the decomposition of H2O2 over just a period of six minutes, and it had already decomposed more than the uncatalyzed H2O2 had done in 24 hours.