Osmosis vs. diffusion is misleading as far as titles go. Both are kinds of passive transport. Passive transport is the gradual movement of molecules from one concentration to another until they are equalized, or at least that’s the shortest definition. Osmosis and diffusion are two ways to accomplish this equilibrium.
Both of these types of passive transport are meant to maintain equilibrium between things like gases, nutrients, water, and some wastes. This is the primary way cells maintain a balance between themselves and extracellular fluids. Both osmosis and diffusion cease once the concentration on both sides of a membrane, like a cell wall, are equalized.
What Exactly is Osmosis?
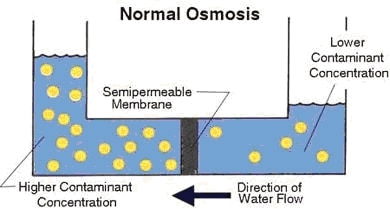
Osmosis is the movement of water, and some other liquids, across a semipermeable membrane such as a cell wall. Osmosis doesn’t require extra energy or pressure to occur. It’s one type of passive transport that allows some cells to move nutrients in or wastes out without using the body’s precious energy reserves. Osmosis moves down the concentration gradient.
Osmosis usually happens when water outside, or inside, a cell is more concentrated and helps move nutrients and wastes in and out of the cell. This is a crucial way cells are fed or grow. Osmosis isn’t just about feeding cells and helping them develop. It can occur between two compartments when the water level in one cell is higher, or a concentration of elements is suspended in water outside a cell.
In mammals, osmosis effects the number of nutrients, typically, inside or outside a cell. Through osmosis, cells maintain a steady flow of nutrients into the cavity for repairs or growth. It’s only the primary way cells get rid of wastes. In plants, osmosis is usually the only way water is absorbed from the ground and sent up the plant to feed cells. Osmosis does not work without water.
What isDiffusion?
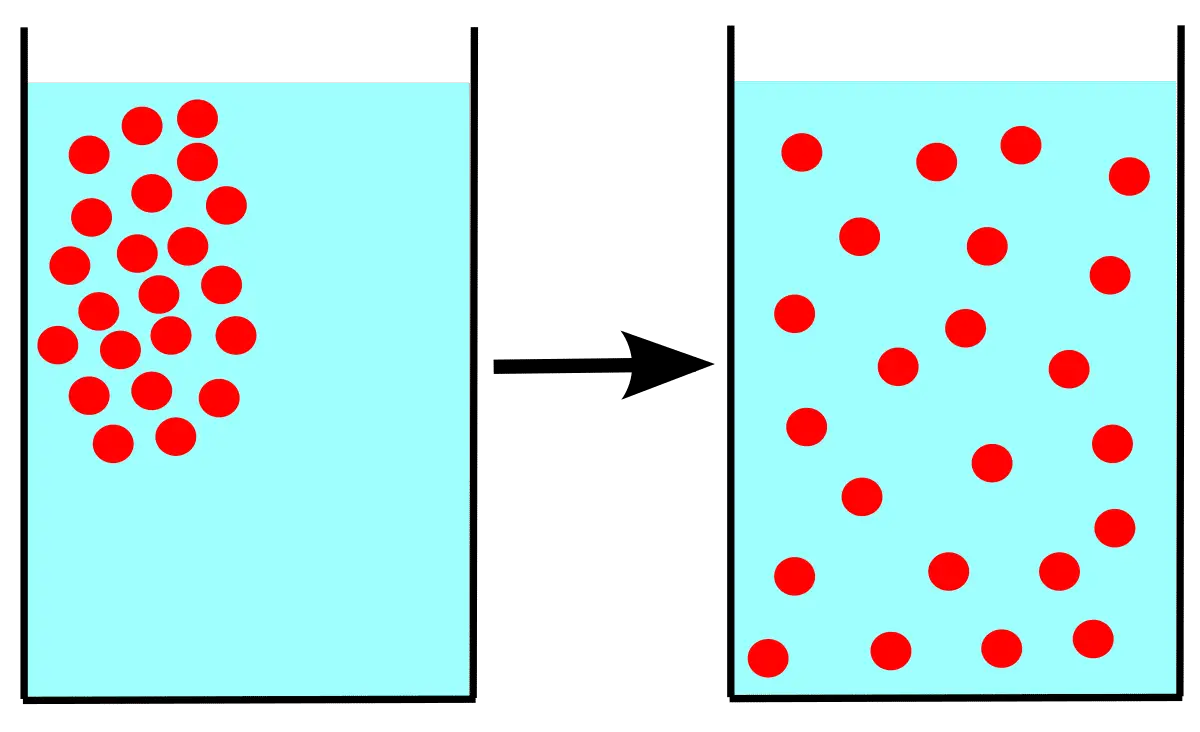
Diffusion is the movement of particles from an area where the particles are dense to an area where the particles are less think. A great example of it is coffee creamer. At first, the creamer is localized to the spot where you poured it in, but after a few minutes, it invades every other part of your coffee cup. Another good example is muddy water mixing with clean water.
Diffusion typically occurs when gases or liquids are directly mixed in varying concentrations. If a membrane or other divider is removed allowing two vapors or liquids to mingle, diffusion is the result once the gas or liquid levels are balanced again. It is significant to body systems responsible for energy production.
Diffusion helps animals and plants maintain life and produce energy. When you breathe, you are using diffusion to keep oxygen flowing in and out of your body. It also helps regulate heat in animals that lack skin pores and sweat glands like dogs. it is essential to plants during their photosynthesis processes. It helps keep their upper levels watered as well.
Osmosis vs. Diffusion Methods
During Osmosis, water molecules pass freely through any semipermeable membrane. This process is spontaneous in both directions until the water concentration on both sides of the layer are equal. The sole purpose of osmosis in cells is to facilitate the movement of nutrients and wastes from outside to inside cells. It regulates the cells hydration during the process as a byproduct.
Anytime the area around the outside of a cell, or a neighboring cell, has a higher concentration of water, osmosis will spontaneously occur until the concentration of water matches on both sides of the cell wall membrane. The same is true if there is more water inside the cell than outside. Osmosis only occurs in the presence of water.
Osmosis also causes cells to swell or deflate based on the amount of water inside or outside the cell. If more water resides outside the cell wall, the cell loses water and tends to shrink. The opposite occurs if more water is outside the cell walls. If the concentration of water remains the same inside and outside, the cell stays the same size and osmosis does not happen. Osmosis always occurs from the lowest to the highest level.
Diffusion is spontaneous just like osmosis but does not require a membrane to pass through. Particles or molecules spread from high concentration areas to low concentration areas. Diffusion creates entropy because it’s random. There’s no measured transfer; it just happens until everything is mixed well. The mixtures that diffuse do become diluted in the process.
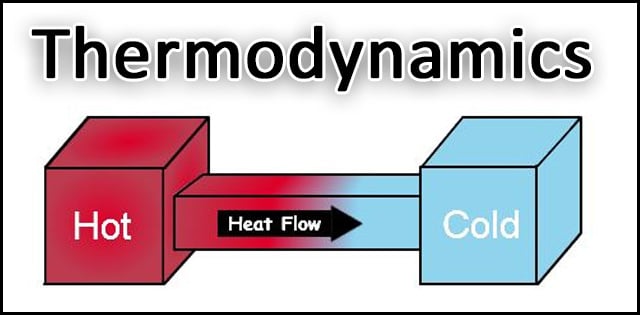
Diffusion follows the Second Law of Thermodynamics because it results in a less concentrated area of energy when it completes. It is the nature of diffusion to introduce randomness and reduce concentrations. It’s the process that allows us to breathe in oxygen and exhale carbon dioxide. The level of oxygen in the air outside our body is higher than it is in our lungs. Diffusion lets us equalize the two.
Osmosis plays a prominent role in the distribution of nutrients and wastes in plants and animals. It helps cells function by supplying them with water and nutrients while removing metabolic wastes from inside the cell. In plants, it takes on additional roles to help the plants get water and nutrients from the soil and move them up the plant.
Diffusion can happen through a semipermeable membrane just like osmosis, but it doesn’t require one to work. While osmosis primarily helps cells move nutrients and wastes around, diffusion helps other particles and molecules such as gases pass through cell walls. Both osmosis and diffusion are necessary to continue life.
The Different Types of Osmosis and Diffusion
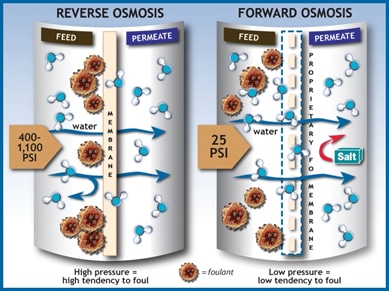
There are only two types of true osmosis, forward osmosis and reverse osmosis. Forward osmosis forces lower concentrated particles to move into higher concentrated areas. This is the primary version of osmosis used to filter things like water in nature. Where regular, or reverse, osmosis tends to push particles around, forward osmosis pulls them in. Forward and reverse osmosis are easy to get confused.
Reverse osmosis works off osmotic pressure. When the concentration of water outside, or inside, a membrane reaches a higher level than its neighbor, osmosis is triggered. If osmosis is possible, it usually prevents diffusion from taking place at the same time. Thus, reverse osmosis can be affected by volumetric and atmospheric pressure to force fluids through a membrane to create a forced filtering process.
There are several different types of diffusion:
- Self-diffusion: measures how much diffusion will occur even with a chemical is at a neutral state.
- Reverse diffusion: very similar to forward osmosis but relates to more particles such as gases.
- Photon diffusion: the movement of light through an object and how the object scatters the light.
- Momentum diffusion: the spread of liquids, mostly, based on the thickness of the liquid. Thicker liquids create higher momentum diffusion.
- Gaseous diffusion: mainly used to enrich uranium for nuclear reactors and weapons.
- Knudsen diffusion: a measure of how a particle reacts to a membrane based on the size of the membrane’s pores and the size of the particle.
- Facilitated diffusion: the spontaneous movement of molecules through a cell membrane at times when osmosis and other forms of diffusion are inhibited.
- Electron diffusion: the movement of electrons to create an electric current.
- Effusion: occurs when a gas is filtered through small holes.
- Surface diffusion: occurs when a dry, powdery substance falls onto the surface of a liquid.
- Collective diffusion: the diffusion of large quantities of particles within a substance that aid each other in moving about the material.
- Osmosis: actually just another form of diffusion.
Examples of Diffusion

Diffusion happens all around and inside us all the time. If you drink tea or coffee, when you add sugar or creamer to them it diffuses until the whole cup is sweeter or creamier. The aroma from air fresheners or cooking food diffuses in the air and invades every room it can reach in your home. These are great examples of passive diffusion since no energy is needed to accomplish diffusion this way.
Plants and animals use diffusion to breathe. Animals draw air into their lungs where it diffuses with the air already in their lungs. This is how we get oxygen into our lungs, and it’s how we get rid of respiratory wastes like carbon dioxide. Carbon dioxide entering a plant’s stomata or oxygen leaving their stomata is how a plant uses diffusion to breathe.
Examples of Osmosis
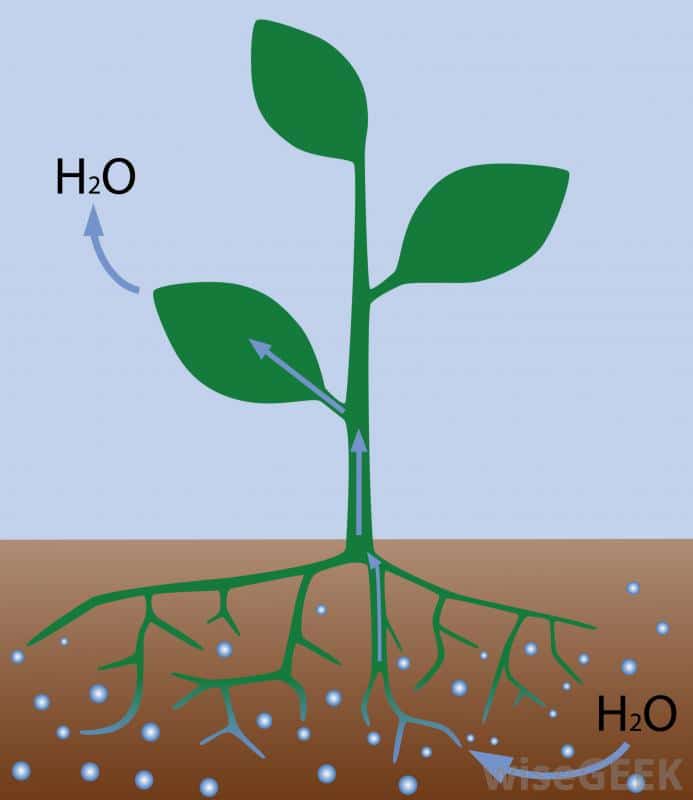
Probably one of the best examples of osmosis is water and nutrients entering a plant’s roots from the soil. Animals use osmosis in a similar way except we absorb nutrients and water throughout our digestive system. Unlike plants, animals eat or drink water and nutrients before they consume them for use by cells to grow and repair themselves.
Some Final Notes
The biggest differences between osmosis and diffusion are how plants and animals use these processes to sustain life. Most kinds of diffusion are similar, and osmosis is technically just another form of diffusion. We use diffusion and osmosis all the time, and most people don’t realize it. It occurs naturally, and it’s manufactured, but it’s necessary for life to exist.


I am impressed by the details that you have article. Thank you for taking the time and sharing this article. It was indeed very helpful and insightful. A good informative post that you have shared and thankful your work for sharing the information. I appreciate your efforts and all the best.
thanks for sharing the article, very helpful