| Diffusion and Osmosis |
Introduction:
Atoms and molecules are constantly in motion. This kinetic energy causes the molecules to bump into each other and move in different directions. This motion is the fuel for diffusion. Diffusion is the random movement of molecules from an area of higher concentration to an area of lower concentration. This will occur until the two areas reach a dynamic equilibrium. When this dynamic equilibrium is reached the concentration of molecules will be approximately equal and there will be no net movement of molecules after this point. The molecules will still be in motion but the concentrations will remain the same.
Osmosis is a special kind of diffusion in which water moves through a selectively permeable membrane. A selectively permeable membrane allows diffusion for only certain solutes (the substance being dissolved) and water, the most common solvent (a dissolving substance). The most common selectively permeable membrane is the cell membrane. Water moves from an area of high water potential to an area of low water potential. Water potential is the measure of free energy of water in a solution and is represented by the symbol ψ (psi). Water potential is affected by two physical factors: the addition of a solute (ψs) and pressure potential (ψp). The addition of solutes to a concentration will lower the water potential of that solute, causing water to move into the area. Water movement is directly proportional to the pressure potential. Water potential can be determined by the equation:
ψ = ψp + ψs
Pure water has a water potential of zero. The addition of solutes will cause the water potential value to be negative, while an increase in pressure potential will cause a more positive water potential value.
There are three relationships that can occur between two solutions. When two solutions have equal solute concentrations, they are isotonic and no net movement of solute occurs. There is also no net movement of water. If the two solutions differ in solute concentrations, they will either be hypertonic or hypotonic. The hypertonic solution has a lower concentration of solute. Water will move out of a hypertonic solution, while solute will move in, (moving up the concentration gradient-similar to water potential). This depends on the selective qualities of the membrane. Pertaining to cells, this will cause the cell to shrivel or become flaccid. The hypotonic solution has a higher concentration of solute, and therefore has less water. This solution will gain water, while losing solute. This movement between the hypotonic and hypertonic solutions will continue until the point of dynamic equilibrium is reached. A hypertonic cell may also undergo plasmolysis. Plasmolysis is the shrinking of the cytoplasm in a plant cell in response to the diffusion of water out of the cell. When a cell is hypotonic it may lyse. In plant cells, it creates turgor pressure against the cell walls keeping the plant from becoming wilted.
Besides osmosis and diffusion, molecules and ions can be moved by active transport. This process includes the use of ATP to drive molecules in or out of a cell. Active transport is generally used to move molecules against a concentration gradient, from an area of low concentration to an area of higher concentration of molecules.
Hypothesis:
In this experiment, diffusion and osmosis will occur until dynamic equilibrium is reached. This experiment is done in a theoretical condition with no other variables affecting the movement of the solute except water potential.
Materials:
Exercise 1A
This exercise requires a 30 cm of 2.5 cm dialysis tubing, 250 ml beaker, distilled water, 2 dialysis tubing clamps, 15 ml of 15% glucose/1% starch solution, 4 pieces of glucose tape, 4 ml of Lugol’s solution (Iodine Potassium-Iodide or IKI), and clock or timer.
Exercise 1B
This experiment requires six strips of 30 cm dialysis tubing, 250 ml beaker, 12 dialysis tubing clamps, distilled water six cups, scale, timer or clock, paper towels, and about 25 ml of each of these solutions: distilled water, .2 M glucose, .4 M glucose, .6 M glucose, .8 glucose, and 1.0 M glucose.
Exercise 1C
This experiment requires a large potato, potato corer (about 3 cm long), 250 ml beaker, paper towel, scale, six cups, knife, and about 100 ml of each of these solutions: distilled water, .2 M glucose, .4 M glucose, .6 M glucose, .8 glucose, and 1.0 M glucose.
Exercise 1D
This experiment requires a calculator, paper, pencil, and graphing paper.
Exercise 1E
This experiment requires onion skin, dye, microscope, slide, cover slip, salt water (15%), and tap water.
Methods:
Exercise 1A
First, soak the dialysis tubing in distilled water for 24 hours. Remove the tubing and tie off one end using the clamp (twist tubing end about 7 times and fold end on self, slide into the clamp). Next, open the other end of the tubing (rubbing end between fingers) and fill it with the glucose/starch solution. Use the glucose tape and record the color change of the tape and the color of the bag. Tie of the end with the tubing clamp (leave empty space, but no air). Fill the beaker with distilled water and add the 4-ml’s of Lugol’s solution, record the color change. Use glucose tap to test for any glucose in the water (record). Set the dialysis tubing in the beaker and let it sit for about 30 minutes. Remove the bag and record the change in water and bag color. Use the last two pieces of glucose tape to measure the glucose in the water and bag and record results.
Exercise 1B
First, soak the dialysis tubing for about 24 hours. Tie off one end of each tube with the clamps. Next, fill each tube with a different solution (distilled water, .2 M glucose, .4 M glucose, .6 M glucose, .8 glucose, and 1.0 M glucose) and tie off the end (leave empty space, but no air). Weigh each tube separately and record the masses. Soak the tubes in separate cups filled with distilled water for about 30 minutes. Remove the tubing, blot dry, reweigh, and record the mass.
Exercise 1C
First, slice the potato into to 3-cm discs. Use the potato corer and core out 24 cores (don’t get any). Weigh 4 cores together and record the mass. Fill each cup with a different solution (distilled water, .2 M glucose, .4 M glucose, .6 M glucose, .8 glucose, and 1.0 M glucose). In each cup put 4 potato cores and let it sit over night. Take out the cores and blot them dry. Record the change in mass. Calculate the information and compare.
Exercise 1D
First, determine the solute potential of the glucose solution, the pressure potential, and the water potential. Then, graph the information given about the zucchini cores.
Exercise 1E
First, prepare a wet mount slide of dyed onion skin. Observe under a light microscope and sketch what the cells. Add a few drops of the salt solution, observe, and sketch the change.
Results:
Exercise 1A
Table 1 Change of Color of Dialysis Tubing and Beaker
|
Solution Color |
Presence of Glucose (Glucose Tape) |
||||
|
Initial |
Final |
Initial |
Final |
||
|
Dialysis Bag |
15% Glucose/1% Starch | Clearish White | Indigo | Dark brown | Mahogany |
|
Beaker |
Water + IKI | Amber | Amber | Yellow teal | Tan-green |
Which substance(s) are entering the bag and which are leaving the bag? What experimental evidence supports your answer?
Glucose is leaving the bag slowly; this is shown buy using the glucose tape in the beaker. Iodine is entering the dialysis bag; this is shown by the change of color in the bag. Water enters the bag; the bag becoming fatter, which shows this.
Explain the results you obtained. Include the concentration differences and membrane pore size in your discussion.
The substances moved and out of the bag, according to the gradient. Some were small substances and moved in and out of the bag quickly and easily. The larger substances, like starch and glucose, were slow or didn’t enter or leave the bag at all.
Quantitative data uses numbers to measure observed changes. How could this experiment be modified so that quantitative data could be collected to show that water diffused into the dialysis bag?
The mass of the bag could be recorded before and after soaking.
Based on your observations, rank the following by relative size, beginning with the smallest: glucose molecules, water, IKI, membrane pores, and starch molecules.
Water→ IKI→ Glucose Molecules→ Membrane Pores→ Starch Molecules
What results would you expect if the experiment started with a glucose and IKI solution inside the bag and only starch and water outside? Why?
The IKI would have left the bag and changed the color of the solution in the beaker. Eventually dynamic equilibrium would be reached and there will be no net movement.
Exercise 1B
Table 2 Dialysis Bag Results: Individual Data
|
Contents of Dialysis Bag |
Initial Mass (g) |
Final Mass (g) |
Mass Difference (g) |
Percent Change in Mass* |
|
a) Distilled Water |
24.0 | 24.2 | 0.2 | .83% |
|
b) 0.2 M |
26.2 | 26.7 | 0.5 | 1.9% |
|
c) 0.4 M |
26.4 | 27.0 | 0.6 | 2.2% |
|
d) 0.6 M |
27.9 | 31.2 | 3.3 | 11.8% |
|
e) 0.8 M |
28.4 | 31.9 | 3.5 | 12.3% |
|
f) 1.0 M |
29.6 | 34.6 | 5.0 | 16.9% |
|
* To Calculate: Percent Change in Mass = Final Mass – Initial Mass Initial Mass * 100
|
Table 3
Dialysis Bag results: Class Data
|
Solution |
Group 1 |
Group 2 |
Group 3 |
Average |
|
a) Distilled Water |
.77% | 1.53% | .83% | 1.04% |
|
b) 0.2 M |
1.86% | 5.30% | 1.9% | 3.02% |
|
c) 0.4 M |
2.4% | 2.22% | 2.2% | 2.27% |
|
d) 0.6 M |
12.54% | 9.75% | 11.8% | 11.36% |
|
e) 0.8 M |
13.07% | 9.64% | 12.3% | 11.67% |
|
f) 1.0 M |
16.55% | 18.98% | 16.9% | 17.48% |
|
Team Members |
Tripp & Stephanie | Hudgens & Kris | Elizabeth & Julie |
7.81% |
Graph 1

Percent Change in Mass of Dialysis Tubing in Glucose Solutions of Different Molarity
Explain the relationship between the change in mass and the molarity of sucrose within the dialysis bags.
They are directly proportional. The percent change of mass goes up as the molarity of the sugar goes up.
Predict what would happen to the mass of each bag in this experiment if all the bags were placed in a 0.4 M sucrose solution instead of distilled water. Explain your response.
There will be no net movement when 0.4 M is in the dialysis bag. When the concentration is above 0.4, the bag will lose water. When the concentration is below 0.4, the bag will gain water.
Why did you calculate the percent change in mass rather than simply using the change in mass?
The volumes of the solutions were not exactly the same.
A dialysis bag is filled with distilled water and then placed in a sucrose solution. The bag’s initial mass is 20g, and its final mass is 18g. Calculate the percent change of mass, showing your calculations in the space below.
Percent Change in Mass = Final Mass – Initial Mass X 100 = 18-20 x 100 = 10%
Initial Mass 20
The sucrose solution in the beaker would have been hypertonic to the distilled water in the bag.
Exercise 1C
Table 4 Potato Core: Individual Data
|
Contents of Beaker |
Initial Mass (g) |
Final Mass (g) |
Mass Difference (g) |
% Change in Mass |
|
a) Distilled Water |
1.8 | 21. | .3 | 16.7 |
|
b) 0.2 M |
1.5 | 1.7 | .2 | 13.3 |
|
c) 0.4 M |
1.5 | 1.8 | .3 | 20 |
|
d) 0.6 M |
1.6 | 1.3 | .3 | -18.75 |
|
e) 0.8 M |
1.4 | 1.1 | .3 | -21.4 |
|
f) 1.0 M |
1.6 | 1.3 | .3 | -18.75 |
Table 5 Potato Core Results: Class Data
|
Contents |
Group 1 |
Group 2 |
Total |
Class Average |
|
Distilled Water |
16.7% | 28.5% | 45.2% | 22.6% |
|
0.2 M Sucrose |
13.3% | 21.4% | 34.7% | 17.35% |
|
0.4 M Sucrose |
20.0% | 14.28% | 34.28% | 17.14% |
|
0.6 M Sucrose |
-18.75% | -20.0% | -38.75% | -19.38% |
|
0.8 M Sucrose |
-21.4% | -26.66% | -48.06% | -24.03% |
|
1.0 M Sucrose |
-18.75% | -21.42% | -40.17% | -20.09% |
Graph 2
Percent Change in Mass of Potato Cores at Different Molarities of Glucose

Exercise 1D
If a potato is allowed to dehydrate by sitting in the open air, would the water potential of the potato cells decrease or increase? Why?
The water potential of the cells will decrease, the osmotic potential will decrease, and the solute will increase. This occurs because the cells have become dehydrated.
If a plant cell has a lower water potential than its surrounding environment, and if pressure is equal to zero, is the cell hypertonic or hypotonic to its environment? Will the cell gain water or lose water? Explain your response.
The environment is hypotonic, so it will gain water. This is because it has less water than the surrounding environment.
In figure 1.5, the beaker is open to the atmosphere. What is the pressure potential of the system?
The pressure potential is zero.
In figure 1.5, where is the greatest water potential?
The dialysis bag.
Water will diffuse out of the bag. Why?
The water will diffuse out because there is higher water potential inside the bag.
Zucchini cores placed in sucrose solutions at 27° C resulted in the following percent changes after 23 hours:
% Change in Mass Sucrose Molarity
20% Distilled Water
10% 0.2 M
-3% 0.4 M
-17% 0.6 M
-25% 0.8 M
-30% 1.0 M
Graph 3
Percent Change in Mass of Zucchini Cores of Sucrose Solutions of Different Molarity

b) What is the molar concentration of solutes with in the zucchini in cells?
About .36 M
Refer to the procedure for calculating water potential from experimental data.
Calculate solute potential (ψs) of the sucrose solution in which the mass of the zucchini cores doesn’t change. Show work.
ψs =-iCRT
ψs =-(1)(0.35)(0.0831)(295)
ψs =-8.580075
ψ =0+ ψs
ψ =0+(-8.580075)
ψ =-8.580075
Calculate the water potential (ψ) of the solutes within the zucchini cores. Show work here.
ψ = ψs + ψp
-8.580075 = ψs + 0
-8.580075 = ψs
What effect does adding solute have on the solute potential component of that solution? Why?
Adding more solute will increase the solute potential and decrease water potential by making it more negative.
Consider what would happen to a red blood cell placed in distilled water:
a) Which would have the higher concentration of water molecules?
Distilled Water
b) Which would have the higher water potential?
Distilled Water
c) What would happen to the red blood cell? Why?
The red blood cells would pull in water and lyse.
Exercise 1E
Prepare a wet mount of a small piece of epidermis of an onion. Observe under 100x magnification. Sketch and describe the appearance of the onion cells.
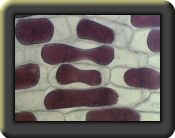
Describe the appearance of the onion cells after the NaCl was added.
The plasma membrane shriveled from the cell wall, causing plasmolysis.
Remove the cover slip and flood the onion with fresh water. Observe and describe what happened.
The onion cells absorbed water and increasing in turgor pressure.
What is plasmolysis?
Plasmolysis is the shrinking of the cytoplasm of a plant cell in response to diffusion of water out or the cell and into a hypertonic solution surrounding the cell.
Why did the onion cell plasmolyze?
The environment became hypertonic to the cell and the water left the cell running with its concentration gradient because of the salt. With all the water leaving the cell, it shrank, leaving behind its cell wall.
In the winter, grass often dies near roads that have been salted to remove ice. What causes this to happen?
The salt causes the plant cells to plasmolyze.
Error Analysis:
Exercise 1A
Error that could have occurred in this experiment is that some of the glucose/starch solution could leaked into the beaker before the dialysis bag was inserted, but after the glucose test.
Exercise 1B
Error that could have occurred in this experiment is that the sugar could mot have been mixed completely with the water or some sugar could have been lost during the mixing. Another mistake could be that the bags were not bloated dry well enough.
Exercise 1C
Error that could have occurred in this experiment is that the sugar could mot have been mixed completely with the water or some sugar could have been lost during the mixing. Another mistake could be that the potato cores were not bloated dry well enough. Also the measuring of the liquids’ volumes may not have been accurate.
Exercise 1D
Errors that could have been made in this exercise could be that the numbers were put in the calculator wrong.
Exercise 1E
Error that could have been made in this exercise could have been that the onion skin could have dried out before the salt water was added, thus affecting the results.
Discussion and Conclusion:
Exercise 1A
In this experiment, the ability of substances to move across a selectively permeable membrane was viewed. A glucose/starch solution was put in the dialysis bag. The glucose molecules leaked out of the bag (learned from before and after test with glucose tape). Using IKI to test for starch, the change in color for the bag only shows that the starch molecules were too large to escape out of the dialysis bag, but the IKI molecule were small enough to enter the bag.
Exercise 1B
In this experiment, the study of hypotonic and hypertonic solution was tested. When the bags were placed in a hypotonic solution, they gained water. This could be viewed by massing the bags before and after the soaking.
Exercise 1C
In this experiment, potato cores were proven to have some sugar in them. When placed in low or no sugar environments, they gained water. When placed in high sugar environments, the cores lost water.
Exercise 1D
In this experiment, all these conclusion made in previous experiments were reinforced with scientific equations.
Exercise 1E
In this experiment, the turgor pressure of a plant cell’s plasma membrane was observed. The plasma membrane gives the cell shape and form. When salt is added, the cytoplasm loses water and causes the plasma membrane to shrink. This causes the plant to wilt.
Concentration gradient and water potential affected all aspects of this experiment. Water potential is used by many scientists to study the effects of different substance on plants, for good or bad. Pressure potential and solute potential are the two main components. Water moves to different areas based on water potential. Water will move from high water potential to low or low water potential to high. This change is called a gradient. Ψs = -iCRT is the formula used for solute potential. Water potential and solute potential are inversely proportional. When water potential goes up, solute potential is low. All these factors affected the results of these experiments.
Plant and animal cells react differently to different environment. A hypertonic solution will cause an animal cell to shrink in size. A hypotonic solution for an animal cell will cause it to lyse. Isotonic solutions are ideal for animal cells. Plant cell’s plasma membrane shrinks if in a hypertonic solution, causing the cell wall to loose shape or plasmolyze. While an isotonic solution is good, it doesn’t provide quite enough support for the cell wall. Hypotonic solutions are the best for plant cells. The plasma membrane presses against the inside of the cell wall giving it a lot of support. Without this pressure, turgor pressure, the plant will wilt and die. These experiments enable us to better understand living things, including our own bodies, and be able to take care of them.
