| Lab. 2 – Enzyme Catalysis |  |
Introduction:
This lab will observe the conversion of hydrogen peroxide to water and oxygen gas by the enzyme catalysis. The amount of oxygen generated will be measured and used to calculate the rate of the enzyme-catalized reaction. Enzymes are proteins produced by living cells. Enzymes act as biochemical catalysts during a reaction, meaning they lower the activation energy needed for that reaction to occur. Through enzyme activity, cells gain the ability to carry out complex chemical activities at relatively low temperatures. The substance in an enzyme-catalyzed reaction that is to be acted upon is the substrate, which binds reversibly to the active site of the enzyme. The active site is the portion of the enzyme that interacts with the substrate. One result of this temporary union between the substrate and the active site is a reduction in the activation energy required to start the reaction of the substrate molecule so that products are formed. In a mathematical equation of the substrate (S) binding with the activation site (E) and forming products (P) is:
E + S —> ES –> E + P
Several ways enzyme action may be affected include:
1) Salt Concentration — For example, if salt concentration is close to zero, the charged amino acid side chains of the enzyme molecules will attract each other. The enzyme will then denature and form an inactive precipitate. If salt concentration is extremely high, the normal interaction of charged groups will be blocked, new interactions will occur, and again the enzyme will precipitate. An intermediate salt concentration such as that of human blood (0.9%) is the optimum for many enzymes.
2) pH of the environment — . The pH of a solution is a logarithmic scale that measures the acidity or H+ concentration in a solution. The scale begins at 0, being the highest in acidity, and ends at 14, containing the least amount of acidity. As the pH is lowered an enzyme will tend to gain H+ ions, disrupting the enzyme’s shape. In turn, if the pH is raised, the enzyme will lose H+ ions and eventually lose its active shape.
3) Temperature — Usually, chemical reactions speed up as the temperature is raised. When the temperature is increased, more of the reacting molecules have enough kinetic energy to undergo the reaction. However, if the temperature goes past a temperature optimum, the conformation of the enzyme molecules is disrupted.
4) Activations and Inhibitors — Many molecules other than the substrate may interact with an enzyme. If such a molecule speeds up the reaction it is an activator, but if it slows the reaction down it is an inhibitor.
The enzyme used in this lab is catalase, which has four polypeptide chains that are composed of more than 500 amino acids each. One function of this enzyme is to prevent the accumulation of toxic levels of hydrogen peroxide formed as a by-product of metabolic processes. Catalase is also involved in some of the many oxidation reactions that occur in the cells of all living things. The primary reaction catalyzed by catalase is the decomposition of hydrogen peroxide to form water and oxygen.
2H2O2 ——-> 2 H2O + O2 (gas)
Without the help of catalase, this reaction occurs spontaneously, but very slowly. Catalase helps to speed up the reaction considerably. In this lab, a rate for this reaction will be determined.
Hypothesis:
The enzyme catalase, under optimum salt conditions, temperature, and pH level will speed up the reaction as it denatures the hydrogen peroxide at a higher rate than normal.
Materials:
Exercise 2A
For the first part of the lab, 10 mL of 1.5% H2O2, a 50-mL glass beaker, and 1 mL of fresh catalase are needed. At the second stage a test tube, a hot water bath, 5 mL of catalase, 10 mL of 1.5% H2O2 are needed. Finally, in the third part, a potato, and10 mL of 1.5% H2O2 are needed.
Exercise 2B
For this experiment, 10 mL of 1.5% H2O2, 1 mL of water, 10 mL of sulfuric acid, two 25 mL beakers, 5-10 mL syringe, potassium permanganate, lab aprons and trays are needed.
Exercise 2C
In this section of the experiment, 20 mL of 1.5% H2O2, two glass beakers, 1 mL of H2O, 10 mL of sulfuric acid, a 5 mL syringe, 5-10 mL of potassium permanganate, and lab aprons and trays are used.
Exercise 2D
In the final part of the lab, 6 plastic cups labeled 10, 30, 60, 120, 180, 360, 6 plastic cups labeled acid, 60 mL of 1.5% H2O2, a 50-mL beaker, 6 mL of catalase extract, two 5-mL syringes, potassium permanganate, a timer (clock), lab aprons and trays are needed.
Methods:
Exercise 2A
Transfer 10 mL of 1.5% H2O2 into a 50-mL glass beaker and add 1 mL of freshly made catalase solution. The fresh catalase should be kept on ice until ready to be used. Observe the reaction. Then transfer 5 mL of purified catalase extract to a test tube and place it in a hot water bath for five minutes. Transfer 10 mL of 1.5% H2O2 into a 50-mL beaker and add 1 mL of the boiled catalase solution, after it has cooled. Observe the changes in the reaction. To demonstrate the presence of catalase in living tissue, cut 1 cubic cm of potato, macerate it, and transfer it into a 50-mL glass beaker containing 10 mL of 1.5% H2O2. Observe the results.
Exercise 2B
Put 10 ml of 1.5% H2O2 into a clean glass beaker. Add 1 mL of H2O. Add 10 mL of sulfuric acid (1.0 M). USE EXTREME CARE IN HANDLING ACIDS. Mix the solution well. Remove a 5 mL sample. Place this 5 mL sample in another beaker, and assay for the amount of H2O2 as follows: Place the beaker containing the sample over white paper. Use a burette or 5 mL pipette to add potassium permanganate a drop at a time to the solution until a persistent pink or brown color is obtained. Remember to gently swirl the solution after adding each drop.
Exercise 2C
To determine the rate of spontaneous conversion of H2O2 to H2O and O2in an uncatalyzed reaction, put about 20 mL of 1.5% H2O2 in a beaker. Store it uncovered at room temperature for approximately 24 hours. Put 10 mL of 1.5% H2O2 into a clean glass beaker (using the uncatalyzed H2O2 that set out). Add 1 mL of H2O2 and then add 10 mL of sulfuric acid (1.0 M). Be careful when using acid. Mix this solution well. Remove a 5 mL sample and place it into another beaker. Assay for the amount of H2O2 as follows: Use a 5 mL syringe to add one drop of potassium permanganate at a time to the solution until it becomes a persistent pink or brown color. Gently swirl the solution after adding each drop. Record all results.
Exercise 2D
If a day or more has passed since Exercise B was performed, a baseline must be reestablish. Repeat the assay from Exercise B and record the results. Compare with other groups to check that results are similar. To determine the course of an enzymatic reaction, how much substrate is disappearing over time must be measured. The first thing to be done is to set up the six cups labeled with times, and the other six, one directly in front of each cup with a time on it. Then put 10 mL of 1.5% H2O2 into the cup marked 10 sec. Add 1 mL of catalase extract to this cup. Swirl gently for 10 seconds using a timer or clock for help. At 10 seconds, add 10 mL of sulfuric acid. Remove 5 mL and place in the cup directly in front of the cup marked 10 sec. Assay the 5 mL sample by adding one drop of potassium permanganate at a time until the solution turns a pink or brown. Repeat the previous steps, with clean cups using the times 30, 60, 120, 180, and 360. Record all results and observations.
Results:
Table 1
Enzyme Activity
| Activity | Observations |
Enzyme activity | The reaction caused oxygen gas as a product, which made the wooden splint glow bright red. |
Effect of Extreme temperature
| Boiling the catalase caused it to denature and it resulted in no bubbling in the solution. |
Presence of catalase | The catalase being present in a living thing (potato), caused an extreme reaction with tons of products (02) produced. |
Table 2
Establishing a Baseline
| Volume | |
Initial reading
| 10 mL |
Final reading
| 6.7 mL |
| Baseline ( final volume – initial volume)
| 3.3 mL Potassium permanganate |
Table 3
Rate of Hydrogen Peroxide Spontaneous Decomposition
| Volume | |
| Initial KMnO4
| 5 mL |
Final KMnO4
| .3 mL |
| Amount of KMnO4 used after 24 hours
| 4.7 mL |
| Amount of H2O2 spontaneously decomposed ( ml baseline – ml after 24 hours) | 1.4 mL |
Percent of H2O2 spontaneously decomposed( ml baseline – ml after 24 hours/ baseline) | 57.6% |
Table 4
Reestablishing a Baseline
| Volume | |
Initial reading
| 5 mL |
Final reading
| .8 mL |
| Baseline ( final volume – initial volume)
| 4.2 mL Potassium permanganate |
Table 5
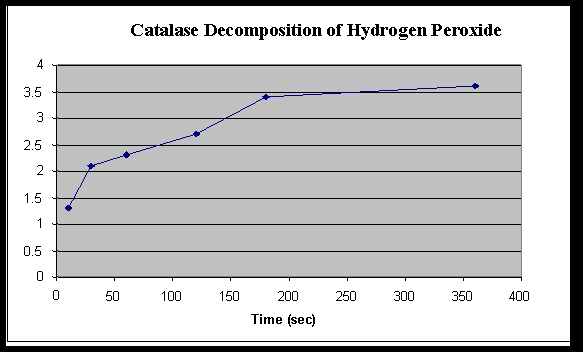
Rate of Hydrogen Peroxide Decomposition by Catalase
Time ( Seconds) | ||||||
| 10 | 30 | 60 | 120 | 180 | 360 | |
| Baseline KMnO4 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 |
| Initial volume KMnO4
| 10 | 10 | 10 | 10 | 10 | 10 |
| Final volume KMnO4
| 7.1 | 7.9 | 8.1 | 8.5 | 9.2 | 9.4 |
| Amount KMnO4 used (baseline – final) | 2.9 | 2.1 | 1.9 | 1.5 | .8 | .6 |
Amount H2O2 used(KMnO4 – initial)
| 1.3 | 2.1 | 2.3 | 2.7 | 3.4 | 3.6 |
Graph 1
![]()
Exercise 2A
1. a) What is the enzyme in this reaction? Catalase is the enzyme in the reaction.
b) What is the substrate in this reaction? The substrate is hydrogen peroxide.
c) What is the product in this reaction? The products are oxygen (gas) and water.
d) How could you show that the gas evolved is oxygen? Using the example of holding a burning wooden splint over the reaction, the splint glows bright red, therefore showing that oxygen is being let out of the solution.
2. How does the reaction compare to the one using the unboiled catalase? Explain the reason for this difference. When the boiled catalase was used, there was no bubbling in the solution, which proved that there was no reaction occurring because the extreme heat had denatured the catalase.
3. What do you observe? What do you think would happen if the potato or liver was boiled before being added to the hydrogen peroxide? The catalase shows a lot of reaction with the potato, causing many bubbles to form in the solution. Also, if the potato were boiled there wouldn’t be any bubbles, because the heat would denature the potato.
Analysis of Results
1. Determine the initial rate of the reaction and the rates between each of the time points. Record the rates in the table below.
| Time Intervals (seconds) | ||||||
| Initial to 10 | 10 to 30 | 30 to 60 | 60 to 120 | 120 to 180 | 180 to 360 | |
| Rates | .13 | .04 | .007 | .007 | .0012 | .0011 |
2. When is the rate the highest? Explain why. The rate is the highest at the beginning of the reaction, because the hydrogen peroxide had been exposed to the air for the least amount of time.
3. When is the rate the lowest? For what reasons is the rate low? At the longer times the rate was the lowest because the peroxide had been exposed to the air longer.
4. Explain the inhibiting effect of sulfuric acid on the function of catalase. Relate this to enzyme structure and chemistry. The sulfuric acid lowered the pH level of the solution, which caused the catalase to denature by gaining hydrogen ions and it stopped the reaction immediately.
5. Predict the effect lowering the temperature would have on the rate of enzyme activity. Explain you prediction. Enzymes work best at optimum temperature, therefore increasing, or in this case, decreasing the temperature would extremely change the rate of the reaction. Lowering the temperature would cause the reaction to slow down.
6. Design a controlled experiment to test the effect of varying pH, temperature, or enzyme concentration. Since the results of room temperature and heated have already been recorded, using catalase that was completely frozen would test the other end of the spectrum as far as temperature goes.
Error Analysis:
Any errors occurring in this experiment could have been caused by misreading of a syringe, miscalculating the data on the tables, or when the 1.5% H2O2 was mixed that was used in almost all parts of the experiment. Also, in Exercise 2D, if the getting the timing just right with all parts of the lab were a source of error in the experiment.
Discussion and Conclusion:
The main purpose of this lab was to show how enzymes can be affected in reactions with other substances by factors such as pH, temperature, and exposure to the surrounding environment. This lab proved that an extreme increase in temperature (boiling) can cause absolutely no reaction by using the boiled enzyme catalase. Also, by using a potato, it shows that catalase is speeding up the decomposition of hydrogen peroxide in living things, helping all living things survive another day.
