| Osmosis through the Cell Membrane of an Egg |
 |
Introduction:
Transport can be either passive or active. Passive transport is the movement of substances across the membrane without any input of energy by the cell. Active transport is the movement of materials where a cell is required to expend energy. In the case of this lab the discussion will be centered on passive transport.
The simplest type of passive transport is diffusion. Diffusion is the movement of molecules from an area of higher to an area of lower concentration without any energy input. Diffusion is driven by the kinetic energy found in the molecules. Diffusion will eventually cause the concentration of molecules to be the same throughout the space the molecules occupy, causing a state of equilibrium to exist.
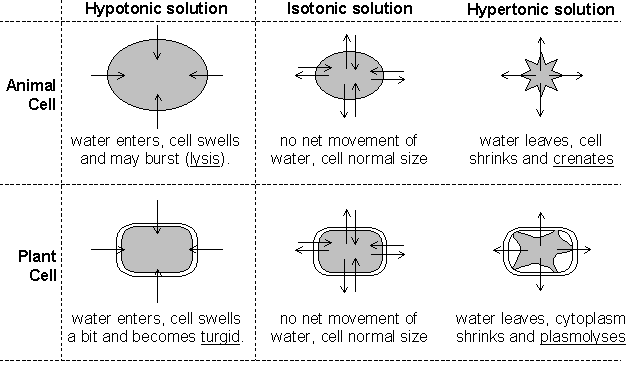
Another type of passive transport is that of osmosis. Osmosis is the movement of water across a semi-permeable membrane. The process by which osmosis occurs is when water molecules diffuse across a cell membrane from an area of higher concentration to an area of lower concentration. The direction of osmosis depends on the relative concentration of the solutes on the two sides. In osmosis, water can travel in three different ways.
If the molecules outside the cell are lower than the concentration in the cytosol, the solution is said to be hypotonic to the cytosol, in this process, water diffuses into the cell until equilibrium is established. If the molecules outside the cell are higher than the concentration in the cytosol, the solution is said to be hypertonic to the cytosol, in this process, water diffuses out of the cell until equilibrium exists. If the molecules outside and inside the cell are equal, the solution is said to be isotonic to the cytosol, in this process, water diffuses into and out of the cell at equal rates, causing no net movement of water.
In osmosis the cell is selectively permeable, meaning that it only allows certain substances to be transferred into and out of the cell. In osmosis, the proteins only on the surface are called peripheral proteins, which form carbohydrate chains whose purpose is used like antennae for communication. Embedded in the peripheral proteins are integral proteins that can either be solid or have a pore called channel proteins. Channel proteins allow glucose, or food that all living things need to live, pass through.
Hypothesis:
In the syrup solution, there will be a net movement of molecules out of the egg, and in the water solution, the molecules will diffuse in and out of the cell at equal rates.
Materials:
The materials used in this lab were 2 fresh eggs in the shell, an overhead marker, 400 ml of water, graduated cylinder, 1 large beaker, 2 medium beakers, 1 small beaker, white vinegar, Karo syrup, distilled water, pencil, paper, lab apron, lab goggles, saran wrap, masking tape, plastic tray, tongs, electronic balance, osmosis lab sheet, and computer.
Methods:
On day 1, measure the masses of both the eggs with the shell. Label 1 beaker vinegar, and then use the graduated cylinder to measure 400 mL of vinegar to put in the labeled beaker. Place both eggs in the solution (place a small beaker on top of the eggs, if necessary) then cover. Let the eggs stand for 24 hours or more to remove the shell.
On day 2, record the observations of what happened to the eggs in the vinegar solution. Carefully, remove the eggs from the vinegar, gently rinsing the eggs off in water. Clean the beakers used for the vinegar solution preparing them for the syrup solution, and then label the 2 medium beakers syrup. Before the eggs are placed in the syrup solution record the mass of both eggs then put it on the datasheet. After that has been done, place the eggs in the beaker, pouring enough syrup to cover the eggs, cover them loosely and let them stand for 24 hours.
On day 3, record the observations of the egg from the syrup solution. Carefully, remove the eggs from the beakers, gently rinsing the syrup off of the eggs. Pour the remaining syrup in the container provided by the teacher. Clean the two beakers used in the syrup solution, preparing them for the water solution. Before the eggs are placed in the water solution record the mass of both eggs then put it on the datasheet. After that has been done, using a graduated cylinder, measure out 200 mL of water for each beaker. Place the eggs in the water solution, cover and let stand 24 hours.
On day 4, record the observations of the egg from the water solution. Carefully remove the eggs from the beakers, gently rinsing them off. Mass both of the eggs. After the teacher has came and looked at the eggs, discard in the proper place.
Results:
| Isotonic Solution |
Hypotonic (Vinegar is acid in Water) |
 |
 |
| Hypertonic |
 |
Table 1- Egg 1 Data
|
Egg mass before added into the solution (g) |
Egg mass after added into the solution (g) |
Observations |
|
Vinegar |
70.8 g (with shell) |
98.0 g (without shell) |
Before the egg was added into the vinegar, it was large, but the after effect was that the egg increased in size and had become hard. After two days, the shell was completely removed. |
|
Syrup |
98.0 g |
65.0 g |
When the egg was removed from the syrup, it had shrunk and it was softer than before it was added into the solution |
|
Water |
65.0 g |
105.3 g |
When the egg was removed out of the water, the color looked of a pale yellow. The water had diffused into the egg, because the egg was larger in size before it was added into the water. |
Table 2- Egg 2 Data
|
Egg mass before added into the solution (g) |
Egg mass after added into the solution (g) |
Observations |
|
Vinegar |
71.6 g (with shell) |
99.1 g (without shell) |
Egg 2s’ mass was greater than egg 1s’ mass before and after it was added into the vinegar solution. The mass had increased some 20 grams with the shell off. |
|
Syrup |
99.1 g |
64.0 g |
The mass of the egg had decreased some 30 grams after it the egg was removed from the syrup solution. The mass of the egg 2 was smaller than the mass of egg1. |
|
Water |
64.0 g |
105.2 g |
The mass of egg 2 had increased some 50 grams after being added into the water solution. The mass of egg 1, though, was larger than the mass of 2 by 1 gram. If the egg would have remained in the water a little while longer, the egg would have probably went through cytolysis. |
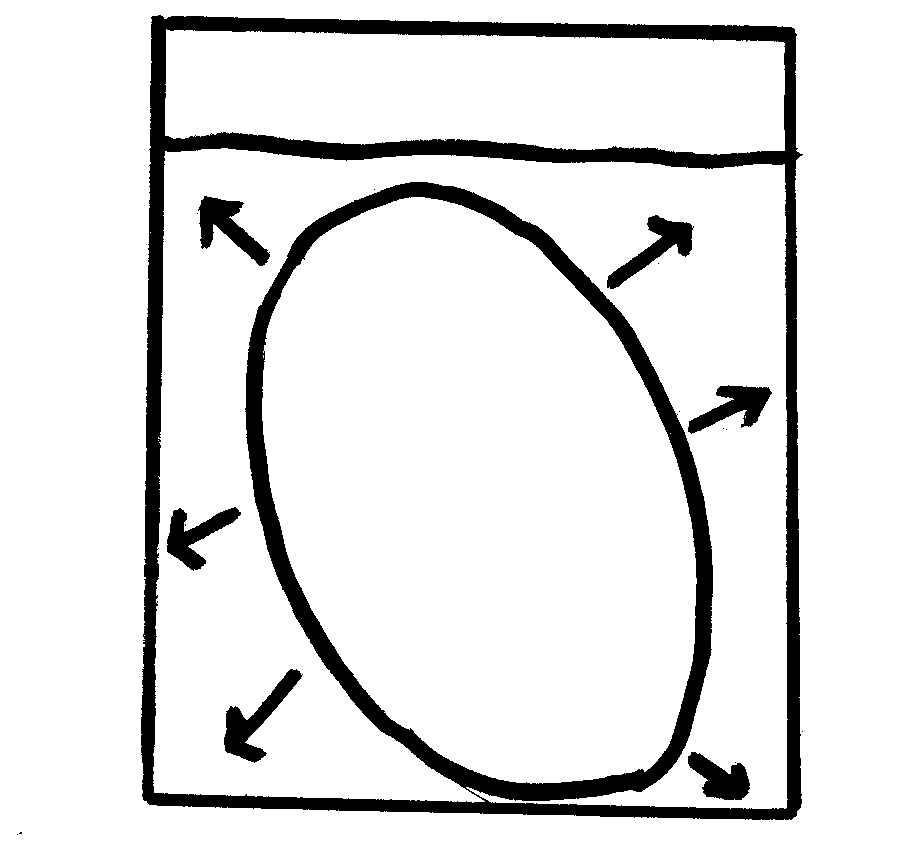
1. When the egg was placed in the water in which direction did the water molecules move? The water molecules moved in the egg.
2. On what evidence do you base this? The molecules moved in, because the size of the egg increased
3. How do you explain the volume of liquid remaining when the egg was removed from the syrup? Since, the cell is selectively permeable, it only allowed a certain amount of the syrup to be present in the cell, just enough to shrink it and also equilibrium was reached..
4. When the egg was placed in the water after being removed from the syrup in which direction did the water move? The water moved in.
5. Why did the water molecules travel better inside the cell than the syrup molecules? The water molecules traveled better into the cell because smaller molecules travel better than other larger molecules.
6. What was the purpose of placing the egg in vinegar? The vinegar solution was only used to remove the shell off the egg.
Error Analysis:
A possible error in this lab occurred by having to leave the egg in vinegar for two days instead of one to remove the shell. This caused the egg to initially take in more water.
Discussion and Conclusion:
Based on the data collected and the results of the experiment, the hypothesis was correct. The egg appeared shriveled after removing it from the syrup because of the movement of water out of the egg. The syrup solution was hypertonic so water moved out of the egg from an area where water was more concentrated to the outside of the egg where water was less concentrated due to the high amount of sugar or solute. The acetic acid in vinegar did remove the shell from the egg, because the egg required two days to completely remove the shell, some water did move into the egg causing its initial mass without the shell to be higher than the egg’s mass with its shell. Whenever the egg was transferred from the syrup to the distilled water, the concentration of water outside the shriveled egg was greater than the water concentration inside the egg; therefore, water moved into the egg until equilibrium was reached. At that point, movement into and out of the egg continued with no net movement of water molecules.
Additional research to see if the egg would have went through cytolysis in another 24 or more hours in the water solution would have been interesting.