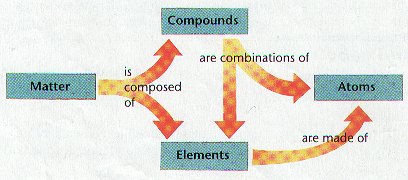
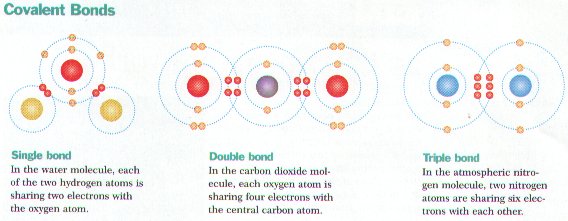
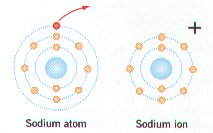
Healthcare is constantly evolving, creating a demand for highly trained professionals who can adapt to new challenges. Those with specialized education in patient care have access to diverse career opportunities, making it easier to find a fulfilling and stable role in the medical field. Whether working in hospitals, clinics, research, or leadership, individuals with the right qualifications can make a lasting impact.
Unlike traditional degrees that take years to complete, some educational paths allow students to gain credentials faster while maintaining high-quality training. This is especially beneficial for those switching careers or looking for an efficient way to enter the nursing industry.
By choosing the right program, individuals can open the door to a world of new possibilities. This article explains how.
Expanding Career Opportunities in Healthcare
A degree in patient care offers more than just a direct path to bedside roles. Those with specialized training can pursue careers in research, administration, public health, and education. The flexibility of this profession allows individuals to find positions that align with their interests and strengths. With healthcare systems growing rapidly, professionals with the right qualifications are needed in various fields. Specializing in a specific area, such as pediatrics, geriatrics, or critical care, can help individuals find rewarding roles beyond traditional hospital settings.
Accelerated Learning
Many people hesitate to start a new career in nursing due to the time commitment required for traditional degree programs. Some specialized educational options allow students to complete their training in a shorter timeframe, making it possible to start working sooner. For instance, 12-month ABSN programs offer an intensive, high-quality curriculum that prepares students for professional roles in a shorter time. These programs combine coursework with hands-on experience, ensuring graduates are ready to enter the workforce with confidence.
Increased Earning Potential and Job Security
Healthcare is one of the most stable industries, with a continuous demand for skilled professionals. Those who complete advanced training often have higher salaries and better job security than those in general roles. According to the Bureau of Labor Statistics, professionals in patient care earn a median salary of $81,220 per year, with higher earnings available in specialized fields. The demand for trained professionals is expected to grow in the coming years, making this a solid career choice for financial stability.
Opportunities for Leadership and Management
Individuals who gain experience in patient care often move into leadership roles. Positions such as department managers, clinical directors, and executives allow professionals to shape healthcare policies and improve patient services. Many facilities seek skilled professionals to take on supervisory roles. Those with strong leadership abilities and advanced training can transition into positions where they oversee teams, manage resources, and contribute to long-term improvements in healthcare settings.
Pathways to Advanced Practice Roles
With additional education, professionals in healthcare can take on more advanced responsibilities. Roles such as medical practitioners, specialists, and healthcare consultants provide greater autonomy and the ability to diagnose, treat, and educate patients. Many organizations encourage professionals to pursue further training to expand their expertise. By continuing their education, individuals can take on specialized responsibilities, helping improve patient outcomes while advancing their careers.
Specialization in Research and Education
A degree in healthcare is not limited to patient care. Many professionals choose to work in clinical research and education, contributing to the development of new treatments, medical procedures, and best practices. Those with expertise in a specialized area can teach future nursing professionals and improve training programs. Hospitals, universities, and research institutions hire professionals with strong medical knowledge to conduct studies and educate others. This path allows individuals to impact the healthcare system by shaping policies, improving patient care techniques, and training the next generation of medical professionals.
Flexibility in Work Environments
Unlike many careers that have fixed schedules and limited work settings, those in healthcare can explore a variety of work environments. Professionals can choose to work in hospitals, clinics, telehealth services, corporate healthcare, or home care. Some may prefer working in a fast-paced emergency setting, while others may thrive in administrative roles or patient education. The ability to transition between different environments helps professionals find a balance that suits their lifestyle and career goals while maintaining job stability.
Contribution to Public Health and Community Wellness
Public health plays a significant role in disease prevention, health promotion, and improving overall community well-being. Professionals trained in patient care can work for government agencies, nonprofit organizations, or community outreach programs to educate people about health risks and provide access to medical resources. Public health initiatives rely on skilled professionals to conduct screenings, design wellness programs, and address health disparities. Those passionate about making a difference outside of clinical settings can find rewarding opportunities in community health roles.
Global Opportunities in Healthcare
The demand for healthcare professionals extends beyond national borders, allowing individuals to explore international job opportunities, medical missions, and travel assignments. Some organizations hire professionals for humanitarian work, disaster relief, and global health programs. Those interested in international healthcare can work with nonprofits, government health agencies, or global medical organizations. This experience helps professionals gain cultural competency, expand their skills, and contribute to improving healthcare access worldwide.
Continuous Learning and Professional Development
The healthcare field constantly evolves, requiring professionals to stay updated with the latest medical research, technology, and patient care advancements. Continuing education, certifications, and specialized training allow professionals to remain competitive and provide the best care possible. Many institutions encourage lifelong learning by offering workshops, certification programs, and professional development courses. Those who actively seek new knowledge and skills have more opportunities to advance in their careers and take on leadership roles.
A specialized nursing degree opens doors to numerous career paths beyond traditional hospital roles. Whether focusing on leadership, research, education, or global health, professionals have the flexibility to shape their careers in meaningful ways. By choosing the right educational program and staying committed to learning, individuals can secure long-term job stability, career advancement, and the ability to make a lasting impact in healthcare. Investing in professional growth ensures a fulfilling and rewarding career in an industry that continues to evolve and expand.


![[Periodic Table]](https://biologyjunction.com/images/periodic.jpg)